The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 24, Issue 1 / June 2020
Research Article Pages:40-48
10.5935/0946-5448.20200008
A Chart Review to Assess the Response of Veterans, Suffering from Tinnitus to Alpha Burst Transcranial Magnetic stimulation
Authors: Alexander Ring, BS, Celeste Crowder, BS, Sharon Link Wyer, PhD, Bill Phillips
PDF
Abstract
Objectives: The purpose of this chart review was to assess the response of veterans suffering from tinnitus to Magnetic EEG/EKGguided resonance therapy and Alpha Burst Stimulation (ABS), while also investigating the safety profile of this therapy combination. EEG/EKG-guided Repetitive Transcranial Magnetic Stimulation (rTMS) delivers high-energy electromagnetic pulses to induce current flow in the neocortex. ABS provides rTMS pulses in short, high-frequency bursts. Materials and Methods: All equipment used to evaluate and treat participants are either FDA-cleared or are exempt from clearance and listed with the United States FDA. Stimulation was delivered with a MagPro R30 and an MCF-B65 butterfly coil. Charts were reviewed from patients who had received a combination of EEG/EKG-guided rTMS and ABS therapy to relieve symptoms of tinnitus. Paired samples t-tests were performed on the Tinnitus Functional Index (TFI) and Neurobehavioral Symptom Inventory (NSI) scales. Treatment logs and therapy notes were reviewed for safety data. Adverse events or side effects were extracted from therapy notes. Linear regression was used to analyze the relationship between number of therapy sessions, and reported patient symptoms. Results: Eighteen of the 23 patients reported significant improvements in tinnitus symptoms. For patients reporting improvements, there was an average 44% reduction in tinnitus symptoms and a 60% reduction in NSI scores following intervention. No patients experienced adverse side effects. The most common side effects were headache and fatigue. Conclusion: Based on the results from this study, noninvasive neuromodulation holds promise as a potential treatment for tinnitus. Additional investigation in controlled studies may be warranted.
Keywords: Tinnitus, neuromodulation, EEG/EKG-guided, rTMS, Alpha-Burst Stimulation (ABS)
Introduction
Tinnitus is characterized by the perceived sensation of sound in the absence of an external stimulus and varies in presentation and volume. Commonly reported phantom sounds include ringing, buzzing, clicking, hissing, and other variants of sound [1]. According to the U.S. Centres for Disease Control, over 50 million Americans experience some form of tinnitus. Approximately two million have extreme and debilitating cases [2]. The origins of tinnitus are nonspecific. Any insult to the auditory pathway may result in tinnitus. Insults may be organic or trauma-related, including infections, neuromas, head and neck injuries, loud noise exposure, or following use of specific medication [3]. Neurological regions and pathways generating tinnitus vary, but generally involve auditory regions. Regional glucose uptake (FDG-PET) was found to be asymmetrically increased in the left auditory cortex, independent of tinnitus’s perceived laterality [4.5]. Whereas most cortical areas activated in the presence of tinnitus are auditory, the involvement of the frontal-parietal awareness network and hippocampal regions reflect the relevance of memory mechanisms in the persistence of tinnitus [6]. Tinnitus-related mechanisms are driven mainly by homeostatic plasticity, involved in altering the balance between excitatory and inhibitory function of the auditory system [7,8]. Injury, insult, and potential disorganization in these areas may result in functional alteration and tinnitus, carrying with it attentional, emotional, and cognitive side effects. Most tinnitus patients present with hearing loss [9]. The measured frequency of this hearing loss correlated with their tinnitus. These findings indicated that tinnitus impacts similar pathways. Disrupted function may be localized to regions involved in frequency-specific auditory processing. Tinnitus could be associated with a lack of auditory processing-specific network synchrony, as reflected in resting patient brain rhythms [10]. Alpha activity is postulated to reflect functional inhibition, and corresponds to reduced local glucose metabolism. When compared to normal hearing controls, MEG/EEG studies showed reduced, stimulation-absent, spontaneous resting alpha-band activity, coupled with an increase in slow-wave delta-band power in temporal regions [11,12]. Reduced alpha activity in sensory regions is a normal cortical response to sound. A general decrease of alpha bandwidth may be related to perceived tinnitus. With the reduction of alpha activity, for tinnitus patients, the auditory pathways are active even in the absence of sound [12,13]. Additionally, increases in resting slow-wave delta activity local to auditory processing regions indicates inhibition of appropriate shifts between excitatory and inhibitory neuronal networks. Targeted neuromodulation and ABS may re-stabilize neural function for tinnitus sufferers [14].
Tinnitus and Military Populations: Tinnitus is especially prevalent in military populations and is currently the number one disability among U.S. Veterans [15]. At the end of the 2014 fiscal year, nearly 1.3 million Veterans received compensation for tinnitus [16]. Despite the high prevalence among Veterans, there are currently no drugs indicated by the FDA to treat tinnitus. Other interventions for tinnitus include Alprazolam, and Deep Brain Stimulation (DBS) [17]. Alprazolam, an anti-anxiety medication, is associated with reductions in the perceived volume of tinnitus; however, many patients reported a sedating effect. DBS is highly invasive and consists of neuronal stimulation via an implanted electrode [18]. In multiple trials and treatments, there were mixed results regarding efficacy. With the exception of DBS, other methods do not address underlying disruptions in neuronal function.
Neuromodulation: Non-invasive neuromodulation, in the form of repetitive transcranial magnetic stimulation (rTMS), was previously investigated as a possible intervention for tinnitus. rTMS induces a brief electro-magnetic field in neuronal tissue, which induces regional current that may bias and modulate endogenous neuronal oscillations [19]. rTMS may excite or inhibit activity in neuronal tissue depending on pulse frequency, with inhibition generally reported at 1-5Hz, and excitation reported at frequencies over 5Hz. Modulation of neuronal activity in auditory cortical areas by rTMS was reported to reduce tinnitus’ perceivable volume and produce therapeutic results with uni-or-bilateral tinnitus [20,21]. Previous studies reported tinnitus suppression with burst rTMS when stimulation was applied at frequencies corresponding to theta (5Hz), alpha (10Hz), and beta (20HZ) frequencies. The variability in response to different stimulation frequencies suggests opportunities for frequency-specific intervention. In this protocol, we reviewed charts of tinnitus patients who underwent a combination of alpha burst rTMS and personalized EEG-EKG guided rTMS [22].
EEG-EKG Guided rTMS: EEG-EKG guided rTMS is neuromodulation in which treatment frequency and location are derived from the patient’s resting EEG and resting heart rate. EEG frequency bands (Delta, Theta, Alpha, Beta) are described as harmonic components. The resting heart rate provides a fundamental frequency from which the expected frequencies within dominant EEG bands are calculated. Calculation of the stimulation rate in EEG-EKG guided rTMS is derived from the higher harmonic frequency, nearest to the individual’s dominant EEG frequency. Location of treatment is the area with highest deviation from a normative database with regard to EEG power distribution, and generally corresponds to stimulation in frontal regions [23]. Application of EEGEKG guided rTMS results in cortical excitability or inhibition, synaptic plasticity, and possibly reinforcement of endogenous EEG oscillations. Changes in symptom severity and cognitive performance were reported with EEG-EKG guided rTMS [23-25]. These therapeutic effects hold promise in the study of neuronal function in normal and pathological states, including tinnitus [20-22].
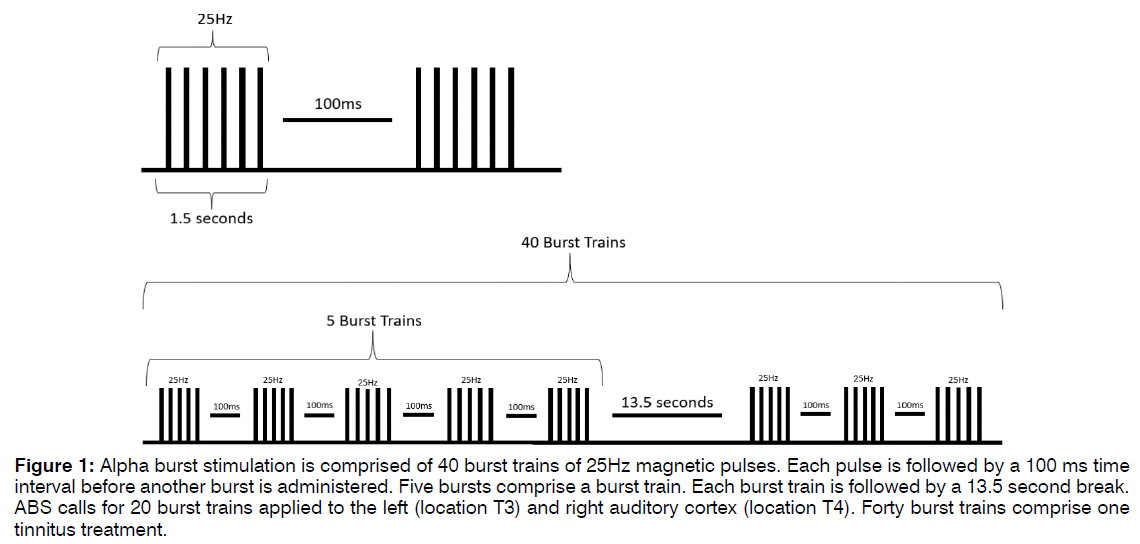
Alpha Burst Stimulation: Alpha Burst Stimulation (ABS) is comprised of short bursts of rTMS pulses with an interval between bursts such that the stimulation frequency falls within the alpha band range, as shown in Figure 1. Five pulses at 25Hz are delivered, followed by an inter-burst interval of 100ms. This burst repeats for five iterations, a total of 1500ms, before a 13.5-second rest period, after which another train of bursts is administered. Forty (40) burst trains are applied, for a total of five minutes of stimulation per application. The procedure is applied first over the right auditory cortex, positioned at EEG location T4, and then repeated over the left auditory cortex at EEG location T3. This approach is designed to mimic the neuronal bursting activity present in auditory regions thought to be responsible for appropriate processing of auditory information. ABS is generally applied for ten sessions, once per day, at least five times per week. Additional treatments are applied at the discretion of the attending clinician. To ensure patient safety, stimulation is delivered between 80-100% of a patient’s motor threshold, at a level that does not cause significant discomfort [23-26]. EEG-EKG guided rTMS and ABS procedures are administered by trained technicians, and patients are evaluated by clinicians and appropriately consented prior to and throughout therapy.
Figure 1: Alpha burst stimulation is comprised of 40 burst trains of 25Hz magnetic pulses. Each pulse is followed by a 100 ms time interval before another burst is administered. Five bursts comprise a burst train. Each burst train is followed by a 13.5 second break. ABS calls for 20 burst trains applied to the left (location T3) and right auditory cortex (location T4). Forty burst trains comprise one tinnitus treatment.
Measures: The Tinnitus Functional Index (TFI) and Neurobehavioral Symptom Inventory (NSI) scores were administered to patients undergoing ABS and EEG-EKG guided rTMS. The Tinnitus Functional Index (TFI), which is the clinical standard, is comprised of eight sections, including: intrusiveness, sense of control, cognitive disruption, sleep disturbance, auditory difficulty, and interference with relaxation, quality of life, and emotional distress [27,28]. These sections can be combined to produce a total TFI score out of a possible 100. The TFI score ranges are as follows: 0-17 not a problem, 18-31 a small problem, 32-53 a moderate problem, 54-72 a big problem, and 73-100 a very big problem. The total scores and eight subsections were analyzed for clinical changes in response to treatment. See Table 3. The NSI is preferred by the Department of Veterans Affairs (VA) to measure post-concussive symptoms in comprehensive traumatic brain injury (TBI) evaluation [29,30]. The NSI is a 22-item self-report questionnaire quantifying patient cognitive wellness over the past two weeks. Patients described on a scale from zero to four the severity of traumatic brain injury symptoms, such as headache, poor coordination, difficulty concentrating, or changes in perception. Based on NSI normative data, scores exceeding 24 are considered to be clinically elevated [27].
Demographics: All patients in this chart review were active or retired US military personnel. Patients were current or past patients of the Brain Treatment Centre San Diego who reported experiencing tinnitus. Eightynine percent of tinnitus patients suffered past TBI. Twenty male and 3 female patients, aged 20-70, were included in records provided for review per protocol.
Materials and Methods
As part of clinical practice, Veterans were asked to complete an NSI and TFI before and after treatment completion. Half of the patients were administered the baseline TFI before treatment, the other half completed their baseline TFI retrospectively, which was shortly after beginning treatment. Number of treatments received, for both EEG-EKG guided rTMS and ABS varied based on clinician judgement and patient response. To measure therapy durability, follow-up TFIs were administered one month following treatment. All patient data was deidentified by the clinic before sharing electronic copies of all patient scales, treatment logs, and therapy notes. Patients were monitored before and during therapy by a licensed clinician. All patients underwent treatment with the understanding that they could stop treatment at any time.
Results
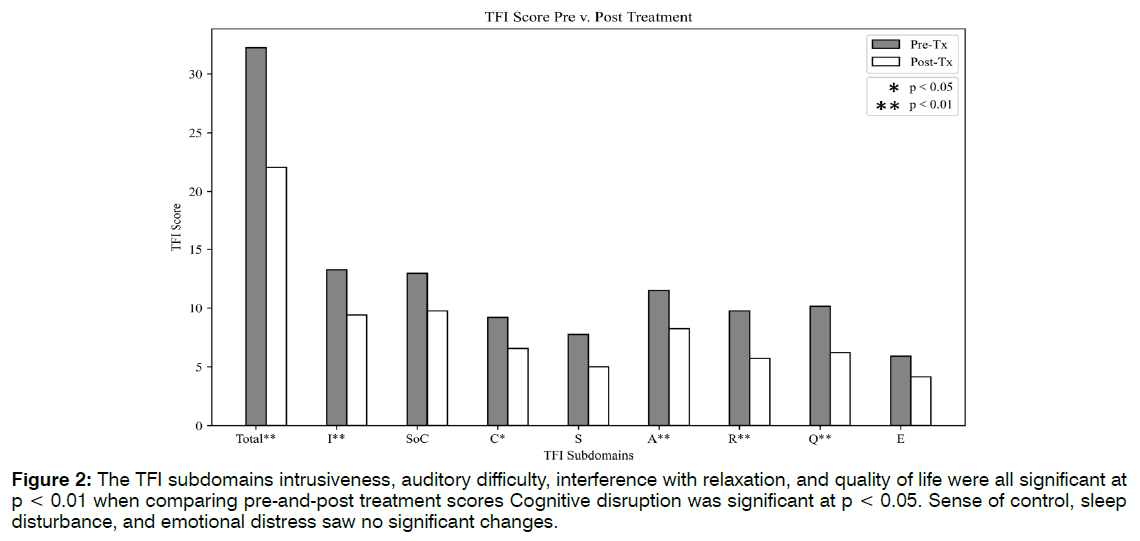
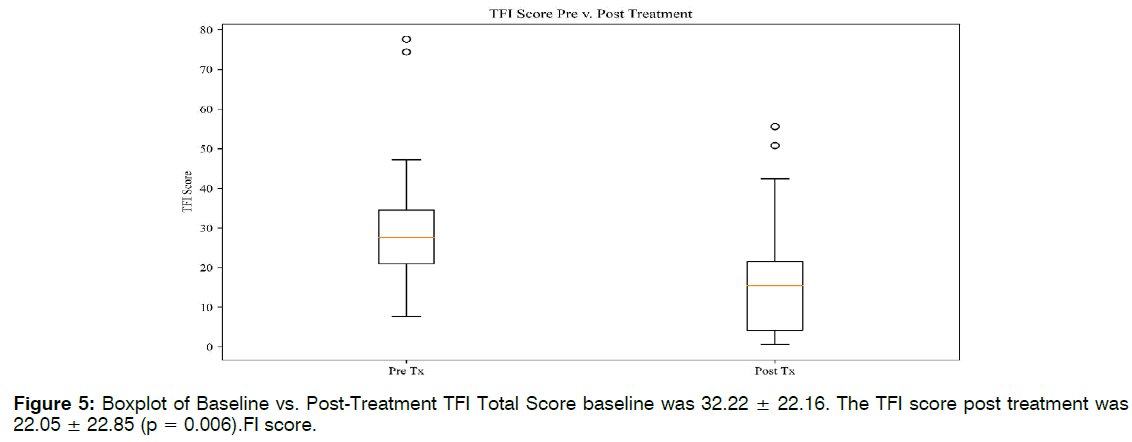
Eighteen of the 23 patients, or 78%, reported a decrease in tinnitus symptoms in both clinician notes and TFI scales. Of the 18 patients reporting improvement, there was a 53% average decrease between baseline TFI and post treatment scores. See Tables 1-3. Twelve of 23 patients’ TFI scores fell into the "not a problem" range after receiving treatment. Eight of these scores dropped one TFI tier as a result of treatment. Four of these improvements spanned two tiers, representing more dramatic shifts. Five of the patients did not shift in TFI categorization, although their numerical scores may have dropped. Two patients reported worsening tinnitus symptoms, and two patients discontinued ABS treatment due to headaches. The average TFI score at baseline was 32.22 ± 22.16. The TFI score post treatment was 22.05 ± 22.85 (p = 0.006). The TFI score at follow-up was 24.11 ± 22.96. Followup scores remained significant from the baseline scores (p = 0.01). It should be noted that the sample size for follow-up TFI was almost half that of baseline (n = 14), due to patient non availability. Five out of the eight TFI subdomains saw significant improvements in Figure 2.
| Group | Mean | SD | Paired T | P Value |
|---|---|---|---|---|
| Baseline | 32.22 | 22.16 | 3.0981 | 0.00592 |
| Post-Treat | 22.05 | 22.85 | - | - |
Table 1: Paired T-Test on Baseline TFI and Post-Treatment Scores.
| Group | Mean | SD | Paired T vs. (N = 14) | P Value |
|---|---|---|---|---|
| Baseline | 31.91 | 22.81 | - | - |
| Post | 22.44 | 24.53 | 2.226 | 0.04432 |
| Follow-Up | 24.11 | 22.96 | 2.8811 | 0.01287 |
Table 2: Paired T-Test on Baseline, Post-Treatment, and Follow-Up TFI Scores.
| Section | Baseline SD | Post Mean | Post SD | Paired T (N=23) |
|---|---|---|---|---|
| Intrusiveness | 7.41 | 9.425 | 6.82 | 0.01 |
| Sense of Control | 8.04 | 9.75 | 8.81 | 0.08 |
| Cognitive Interference | 6.89 | 6.55 | 7.54 | 0.05 |
| Sleep Disturbance | 9.3 | 5 | 6.6 | 0.15 |
| Auditory Interference | 7.57 | 8.25 | 8.55 | 0.01 |
| Relaxation Interference | 7.77 | 5.7 | 7.23 | 0.003 |
| Quality of Life | 9.31 | 6.2 | 8.85 | 0.01 |
| Emotional Distress | 6.9 | 4.15 | 4.15 | 0.96 |
| TFI Total | 22.16 | 22.05 | 22.85 | 0.006 |
Table 3: Paired T-Test on Baseline and Post-Treatment TFI Subdomain Scores.
Figure 2: The TFI subdomains intrusiveness, auditory difficulty, interference with relaxation, and quality of life were all significant at p < 0.01 when comparing pre-and-post treatment scores Cognitive disruption was significant at p < 0.05. Sense of control, sleep disturbance, and emotional distress saw no significant changes.
Post-Treatment: Follow-up TFI’s were collected one month after treatment completion. There were no significant differences between post treatment TFI and the follow-up (p = 0.66), both for the TFI total and each subdomain. These findings suggested that patient relief was sustainable after ceasing treatment. It is worth noting that 10 of the 14 patients who completed a follow-up TFI observed an increase in tinnitus symptoms post-treatment completion. It should also be noted that of the 14 followup TFI scores, seven of those scores were in the "not a problem" range, as defined by the TFI. Because half of the TFI categorizations remained in the “not a problem” range at follow-up, we can assume any changes following treatment completion were minimal.
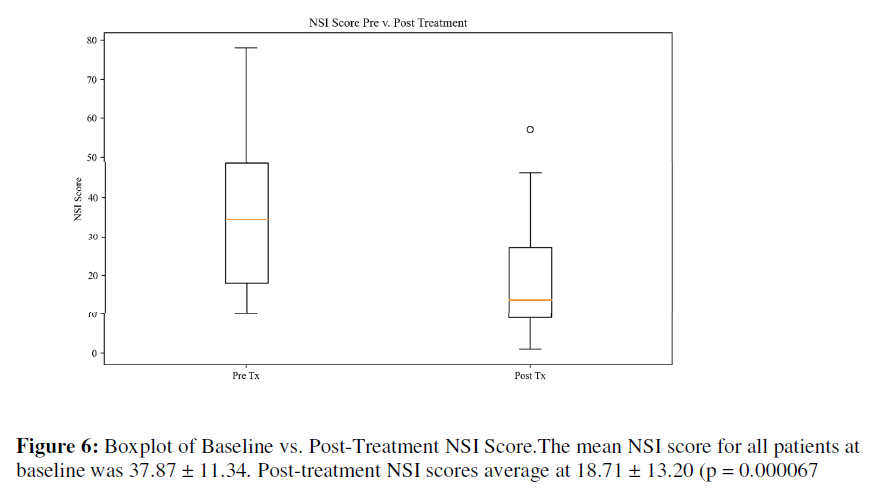
NSI: The mean NSI score for all patients at baseline was 37.87 ± 11.34. Post-treatment NSI scores average at 18.71 ± 13.20 (p = 0.000067), as seen in Table 4. Based on normative NSI data for [30] Veterans with mild TBI, the baseline score began in the top 1% and dropped to the thirtieth percentile All but one patient saw a reduction in the NSI score.
| Group | Mean | SD | Paired T | P Value |
|---|---|---|---|---|
| (N = 23) | ||||
| Baseline | 37.87 | 22.81 | 4.8994 | 0.00006723 |
| Post-Treatment | 18.71 | 13.2 | - | - |
Table 4: Paired T-Test on Baseline and Post-Treatment NSI Scores.
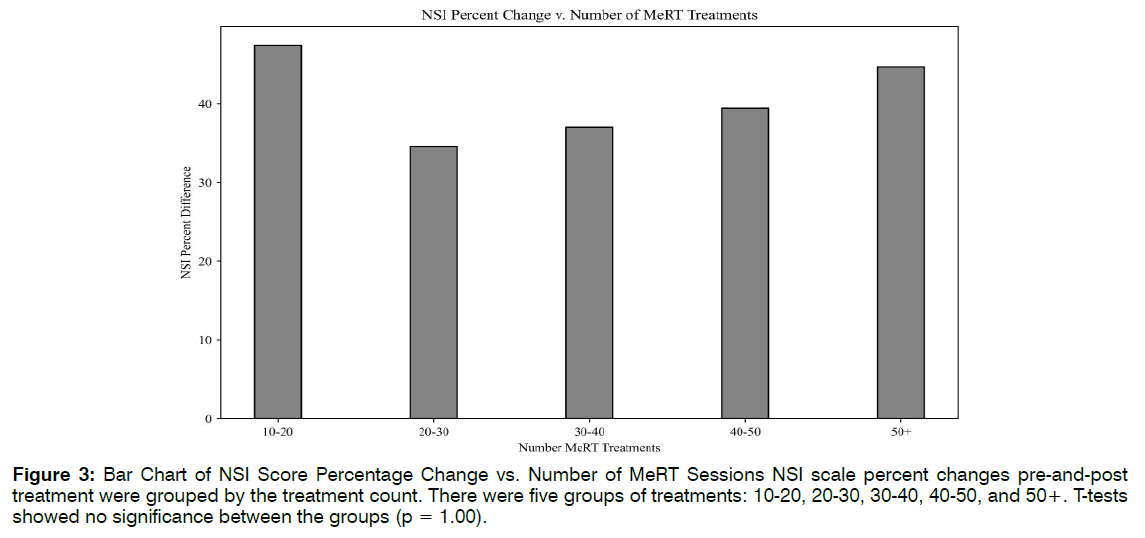
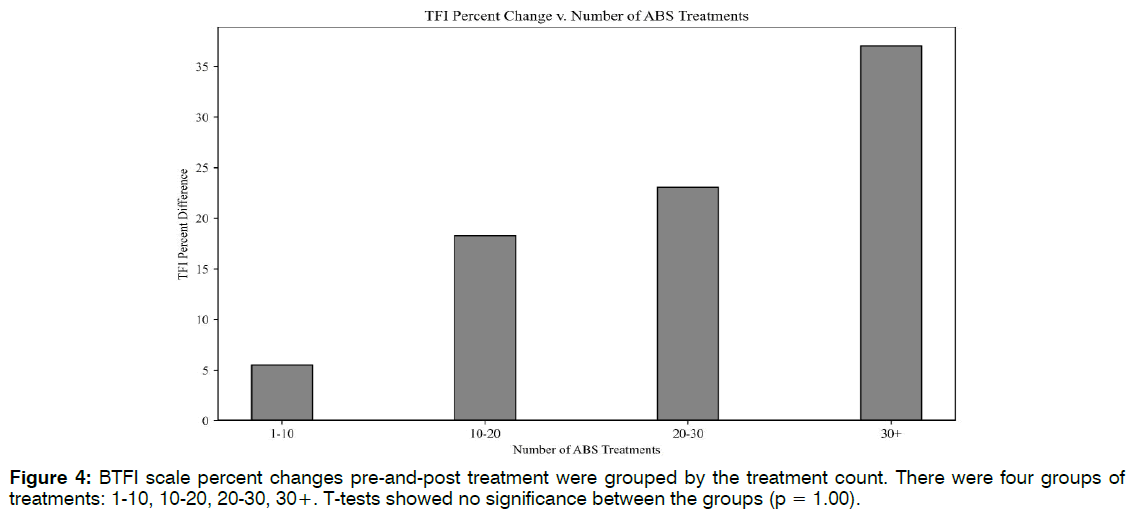
Number of Treatments: Linear regression lines were used to explore the relationships between the number of EEG-EKG guided rTMS treatments and NSI scale changes (R2 = 0.07, F(1,18) = 1.39, p = 0.25), and the number of ABS treatments to TFI improvement (R2 = 0.04, F(1,30) = 1.36, p = 0.24). There were no linear correlations for either, suggesting that higher treatment count may not translate to more robust clinical response past an initial period of therapy. To potentially identify an optimal treatment number, clinical scale percent differences were grouped by their treatment count. There were five groups of treatments:10-20, 20-30, 30-40, 40- 50, and 50+. T-tests showed no significance between the groups (p = 1.00). Figure 3 revealed that patients saw the greatest NSI improvement (47%) between 10- 20 EEG-EKG guided rTMS sessions. This increase does not differ much from the 44% increase seen at 50+ sessions. The same analysis was performed for ABS treatment number and TFI percent differences, with appropriate adjustments made to the treatment count groupings. Though differences were not significant, there was a trend of higher ABS count and greater TFI improvement. Figure 4 indicated that the greatest changes in tinnitus were seen after 20 ABS treatments Figure 4.
Figure 3: Bar Chart of NSI Score Percentage Change vs. Number of MeRT Sessions NSI scale percent changes pre-and-post treatment were grouped by the treatment count. There were five groups of treatments: 10-20, 20-30, 30-40, 40-50, and 50+. T-tests showed no significance between the groups (p = 1.00).
Retrospective TFI: Retrospective reporting lends itself to potential measurement error and bias. To address the issue of retrospective reporting, we separated the data for those who had a retrospective baseline TFI (Group 1), and those who completed the scale before beginning treatment (Group 2). The results for the independent groups were synonymous with original findings. Both groups saw significant differences between the preand post-treatment TFI scores, with p = 0.01 and p = 0.05, respectively. There were no significant differences between Group 1 and 2 baseline TFI scores (p = 0.08). These analyses indicate that the chart review findings cannot be discredited due to retrospective report bias Figure 5.
Side Effects: The most common side effects of rTMS are local discomfort at the site of treatment, headaches, and neck pain (due to head positioning while receiving treatment) [31]. These known side effects have been reported to last no longer than one-hour following treatment [32]. Side effects observed during this clinical chart review were considered mild by FDA standards, and were consistent with those reported in similar applications of rTMS. No patients experienced unusual discomfort and no serious adverse events, such as seizure, occurred. Out of the 23 patients who completed both pre-and-post TFI’s, 65% reported mild headache following treatment. For the majority of patients experiencing headaches (n = 13), symptoms subsided after an average of 11.75 treatments, which was roughly two weeks’ time. One patient reported intermittent headaches throughout treatment that did not resolve; however, the headaches were not intrusive enough to discontinue. Sixty-one percent of patients reported feeling fatigued, 39% reported an increase in anxiety, and 26% reported feeling emotionally volatile. For most patients, these side effects were resolved after two weeks’ time, attributing the emotional changes to a new therapy routine and nerves surrounding treatment expectation. Thirty percent reported that their tinnitus worsened before it improved. Of the patients who reported their tinnitus worsening, these reports occurred early on in treatment, likely as a result of cortical reorganization and attention to individual symptom severity [7]. The seven reports of tinnitus increasing in volume led to eventual improvement, either volume reduction, or complete tinnitus resolution. These resolutions occurred after an average of 16.23 treatments, roughly three weeks’ time. Conclusion two of the 23 patients, there were reports of blurred vision, dizziness, and eye twitching. For both patients, these symptoms occurred after intense workout and may be the result of low-blood sugar, unrelated to EEGEKG guided rTMS or ABS treatment. These patients, who discontinued treatment, reported headaches brought about by ABS as well as physical discomfort at the stimulation site. Therapy notes specified a dull pain at T5, the treatment site, and feelings of increased cranial pressure
Discussion
Tinnitus is extremely prevalent in military populations and is positively correlated with depression, anxiety and TBI [33-36]. In this chart review, we assessed the response of Veterans suffering from tinnitus to personalized neuromodulation in the form of EEG-EKG guided rTMS and ABS in a clinical setting. Patients with tinnitus show common deficits in resting network function, specifically within the alpha range. Non-invasive neuromodulation provides a unique approach that allows for patient specific excitation of tissue at deficit. Following daily administration of combined EEG-EKG guided rTMS and ABS, response to therapy was greatest between 10-20 sessions. The noted response may result from the formation of longterm potentiation of network function matching delivered stimulation. As stimulation parameters mimicked alpha oscillations, a reinforcement of these oscillations may have shifted neuronal bursting from slower frequency rhythms to faster rhythms. Future studies may integrate EEG and FDG-PET to appropriately capture and monitor shifts in local glucose metabolism and alpha density in addition to patient response on clinical scales. Given the small sample size, lack of a control group, and broad range of response, the observed benefit of ABS for tinnitus may be overestimated. Though predominately positive, percent of improvement varied between patients. Further research is necessary to determine how different variables correspond to therapeutic response, including number of treatments, patient age, and tinnitus etiology. We found no significant differences between patient age group and percentage change (p = 0.25); however, the trend suggested the younger the patient was, the more dramatic their response to treatment. Similarly, with number of treatments, there was no significance between groups (p = 0.24), but there was an observable trend. In examining clinician notes and patient scale changes, average symptom improvements occurred at 15-20 treatments for both EEG-EKG guided rTMS and ABS. Consistent with generally reported rTMS side effects, EEG-EKG guided rTMS and ABS side effects, such as headache and emotional volatilely, also subsided after 10- 20 treatments. Future trials may target 10-20 treatments in determining sensitivity to treatment response shown in Figure 6.
Study Limitations
In addition to small sample size and lack of a proper control, this chart review is subject to Survey Non-Response Bias [37]. Half of patients who completed baseline and post-treatment TFI scales did not complete a followup TFI. Results should be considered preliminary until more generalizable data may be reported
Conclusion
Results from this chart review showed that combined ABS and EEG-EKG guided rTMS may be conducive in tinnitus intervention. While this treatment revealed promising results, with a reasonable safety profile, additional double-blind sham-controlled research is necessary to appropriately characterize therapeutic response. Neuromodulation, in the form of EEG-EKG guided rTMS and ABS, may modify the organization and inhibition in resting state network function, consequently impacting tinnitus.
Conflict of Interest
The authors declare no potential conflict of interest on publishing this paper.
References
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in Cognitive Sciences. 2012;16:606-17.
- Control CFD. National Health and Nutrition Examination Survey. 2013.
- Gordon JS, Griest SE, Thielman EJ, Carlson KF, Helt WJ, Lewis MS, et al. Audiologic characteristics in a sample of recently-separated military Veterans: The Noise Outcomes in Servicemembers Epidemiology Study (NOISE Study). Hearing Research. 2017;349:21-30.
- Lanting CP, De Kleine E, Van Dijk P. Neural activity underlying tinnitus generation: Results from PET and fMRI. Hearing Research. 2009;255:1-13.
- Langguth B, Schecklmann M, Lehner A, Landgrebe M, Poeppl T, Kreuzer P, et al. Neuroimaging and Neuromodulation: Complementary Approaches for Identifying the Neuronal Correlates of Tinnitus. Frontiers in Systems Neuroscience. 2012;6:1-5.
- Schaette R, McAlpine D. Tinnitus with a Normal Audiogram: Physiological Evidence for Hidden Hearing Loss and Computational Model. Journal of Neuroscience. 2011;31:13452-7.
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proceedings of the National Academy of Sciences. 2011;108:14974-9.
- Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. European Journal of Neuroscience. 2006;23:3124-38.
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neurosciences. 2004;27(11):676-82.
- Eggermont JJ, Tass PA. Maladaptive Neural Synchrony in Tinnitus: Origin and Restoration. Frontiers in Neurology. 2015;6:1-2.
- Song JJ, De Ridder D, Schlee W, Van De Heyning P, Vanneste S. “Distressed aging”: the differences in brain activity between early- and late-onset tinnitus. Neurobiology of Aging. 2013;34:1853-63.
- Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus Perception and Distress Is Related to Abnormal Spontaneous Brain Activity as Measured by Magnetoencephalography. PLoS Medicine. 2005;2:e153.
- Lind L, Rolphj S, Hariri AR. Evidence for reactive magnetic 10-Hz rhythm in the human auditory cortex. NeurosciLett. 1997;222:111-4.
- Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJF. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends in Neurosciences. 2005;28:325-33.
- Henry JA, Griest SE, Blankenship C, Thielman EJ, Theodoroff SM, Hammill T, et al. Impact of Tinnitus on Military Service Members. Military Medicine. 2019;184:604-14.
- Moring JC, Peterson AL, Kanzler KE. Tinnitus, Traumatic Brain Injury, and Posttraumatic Stress Disorder in the Military. International J of Behavioral Medicine. 2018;25:312-21.
- Folmer RL, Theodoroff SM, Hal Martin W, Yongbing S. Experimental, Controversial, and Futuristic Treatments for Chronic Tinnitus. Journal of the American Academy of Audiology. 2014;25(1):106-25.
- Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus Nerve Stimulation for Treatment-Resistant Depression: A Randomized, Controlled Acute Phase Trial. Biological Psychiatry. 2005;58:347-54.
- Taghva A, Silvetz R, Ring A, Kim KY, Murphy KT, Liu CY, et al. Magnetic Resonance Therapy Improves Clinical Phenotype and EEG Alpha Power in Posttraumatic Stress Disorder. Trauma Mon. 2015;20:e27360.
- Theodoroff SM, Lewis MS, Folmer RL, Henry JA, Carlson KF. Hearing Impairment and Tinnitus: Prevalence, Risk Factors, and Outcomes in US Service Members and Veterans Deployed to the Iraq and Afghanistan Wars. Epidemiologic Reviews. 2015;37:71-85.
- De Ridder D, Heijneman K, Haarman B, van der Loo E. Tinnitus in vascular conflict of the eighth cranial nerve: a surgical pathophysiological approach to ABR changes. Prog Brain Res. 2007;166:401-11.
- Corlier J, Carpenter LL, Wilson AC, Tirrell E, Gobin AP, Kavanaugh B, et al. The relationship between individual alpha peak frequency and clinical outcome with repetitive Transcranial Magnetic Stimulation (rTMS) treatment of Major Depressive Disorder (MDD). Brain Stimul. 2019;12(6):1572-8.
- Boroojerdi B, Meister IG, Foltys H, Sparing R, Cohen LG, Töpper R. Visual and motor cortex excitability: a transcranial magnetic stimulation study. Clinical Neurophysiology. 2002;113:1501.
- John ER. Neurometrics: clinical applications of quantitative electrophysiology. . Hillsdale, N.J.1977.
- De Ridder D, De Mulder G, Verstraeten E, Van der Kelen K, Sunaert S, Smits M, et al. Primary and secondary auditory cortex stimulation for intractable tinnitus. ORL J Otorhinolaryngol Relat Spec. 2006;68:48-54.
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1998;108:1-16.
- Soble JR, Silva MA, Vanderploeg RD, Curtiss G, Belanger HG, Donnell AJ, et al. Normative Data for the Neurobehavioral Symptom Inventory (NSI) and Post-Concussion Symptom Profiles Among TBI, PTSD, and Nonclinical Samples. Clinical Neuropsychologist. 2014;28:614-32.
- Henry JA, Griest S, Thielman E, McMillan G, Kaelin C, Carlson KF. Tinnitus Functional Index: Development, validation, outcomes research, and clinical application. Hearing Research. 2016;334:58-64.
- Ashendorf L. Neurobehavioral symptom validity in U.S. Department of Veterans Affairs (VA) mild traumatic brain injury evaluations. Journal of Clinical & Experimental Neuropsychology. 2019;41:432-41.
- Tyler M, Skinner K, Prabhakaran V, Kaczmarek K, Danilov Y. Translingual Neurostimulation for the Treatment of Chronic Symptoms Due to Mild-to-Moderate Traumatic Brain Injury. Archives of Rehabilitation Research and Clinical Translation. 2019;1:100026.
- Costello A. Repetitive Transcranial Magnetic Stimulation (rTMS) Systems - Class II Special Controls Guidance for Industry and FDA Staff: US. Food and Drug Administration; 2011;2:1-6.
- Tarapore PE, Picht T, Bulubas L, Shin Y, Kulchytska N, Meyer B, et al. Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clinical Neurophysiology. 2016;127:1895-900.
- Folmer RL. Forget auditory nerve compression as a treat-able cause for tinnitus. Neurol Neurosurg Psychiatry. 2010.
- Folmer RL, Griest SE, Meikle MB, Martin WH. Tinnitus severity, loudness, and depression. Otolaryngology–Head and Neck Surgery. 1999;121:48-51.
- Folmer RL, Griest SE. Tinnitus and insomnia. Am J Otolaryngol. 2000;21:287-93.
- Kreuzer PM, Landgrebe M, Frank E, Langguth B. Repetitive Transcranial Magnetic Stimulation for the Treatment of Chronic Tinnitus After Traumatic Brain Injury. Journal of Head Trauma Rehabilitation. 2013;28:386-9.
- Martikainen P, Laaksonen M, Piha K, Lallukka T. Does survey non-response bias the association between occupational social class and health Scandinavian Journal of Public Health. 2007;35:212-5.
Department of Department of Independent Research Writer, University of Irvine Center, USA
Send correspondence to:
ASharon Link Wyer
Department of Department of Independent Research Writer, University of Irvine Center, USA, E-mail: Sharon.Link@sharonlinkphd.com
Phone: +11949-878-1985
Paper submitted on May 22, 2020; and Accepted on June 05, 2020
Citation: A Chart Review to Assess the Response of Veterans, Suffering from Tinnitus to Alpha Burst Transcranial Magnetic. 24(1):40-46.