The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 26, Issue 1 / June 2022
Original Paper Pages:20-26
10.5935/0946-5448.20220004
Evaluation of Medial Olivocochlear Neural Efferent Pathway in Tinnitus Perception in Normal-hearing Individuals
Authors: Amitkumar Tayade, Denise Tucker
PDF
Abstract
Objective: The objective of this study is to investigate a possible role of the Medial Olivocochlear (MOC) efferent neural pathway and neural connections responsible for tinnitus generation in silence/sensory deprivation.
Design: By placing normal hearing participants in a sound booth for 10 minutes, silence/sensory deprivation was created. This offered assessment of MOC neural pathway in normal hearing participants in silence. Hyperactivity of MOC neural pathway was assessed by its more suppressive effect on Transient Otoacoustic Emissions (TEOAEs) in silence. The required auditory measurements were recorded in the sound booth using recommended diagnostic protocols to ensure the effect of ‘only silence’ on auditory structures. TEOAE were recorded from the right ear and suppression was measured by placing noise in the left ear. Fifty-eight normal hearing male individuals between age 18-35 years were recruited as participants in this study.
Results: Approximately, forty-one percent of the participants perceived some type of tinnitus during/after 10 minutes of silence. No statistically significant difference was found in the total TEOAE amplitude and TEOAE suppression amplitude before and after ten minutes of silence. Post silence total TEOAE suppression between tinnitus perceiving and non-perceiving tinnitus participants were not statistically significantly different.
Conclusion: These results suggest that the medial olivocochlear efferent pathway or lower brain stem area does not appear to play a role in the emergence of temporary tinnitus in silence however indicate the involvement of higher central auditory nervous system structures in perception of the tinnitus which support the well-accepted notion that tinnitus is the central auditory processing phenomenon.
Keywords: Silence, Transient Otoacoustic Emissions, Contralateral suppression, Auditory sensory deprivation, Hyperactivity.
Introduction
Tinnitus is the sound perception in the absence of an external auditory stimulus [1]. According to American Tinnitus Association, Approximately, 50 million people in the United States experience tinnitus, 20 million struggles with chronic tinnitus, and two million are completely disabled from it [2]. Currently, there is no medical cure for tinnitus. Animal studies examined the pathophysiology of the auditory system such as increased spontaneous firing rate (hyperactivity) in the auditory nerve fibers [3] and dorsal cochlear nucleus [4] because of cochlear damage. Lack of sensory input due to cochlear damage alters the neural physiology in the auditory pathway and results in rapid seemingly irreversible changes in the auditory system [5-8]. Therefore, tinnitus seems to be associated with greater neural activity in the central auditory structures due to less neural excitation in the periphery of the ascending auditory pathway [9].
The efferent auditory pathway is the part of descending central auditory pathway, starts in the auditory cortex and terminates in the cochlea [10]. The Olivocochlear bundle (OCB), a part of efferent auditory pathway, is located within the brainstem and terminates inside the cochlea [10]. One part of the OCB, the medial olivocochlear (MOC) fibers, is the thick and myelinated project predominantly to the contralateral cochlea and terminate at the base of the outer hair cells [10]. The MOC neural efferent pathway has been studied the most because of the ease with which it can be stimulated electrically and acoustically [11]. Upon activation, MOC fibers inhibit the outer hair cell activity resulting in the decreased (suppressed) otoacoustic emission levels. Considering the neural connections between outer hair cells (OHCs), auditory nerve fibers, cochlear nucleus, MOC (Ipsilateral and contralateral), and MOC fibers back to OHCs, the MOC efferent pathway might contribute to the perception of tinnitus.
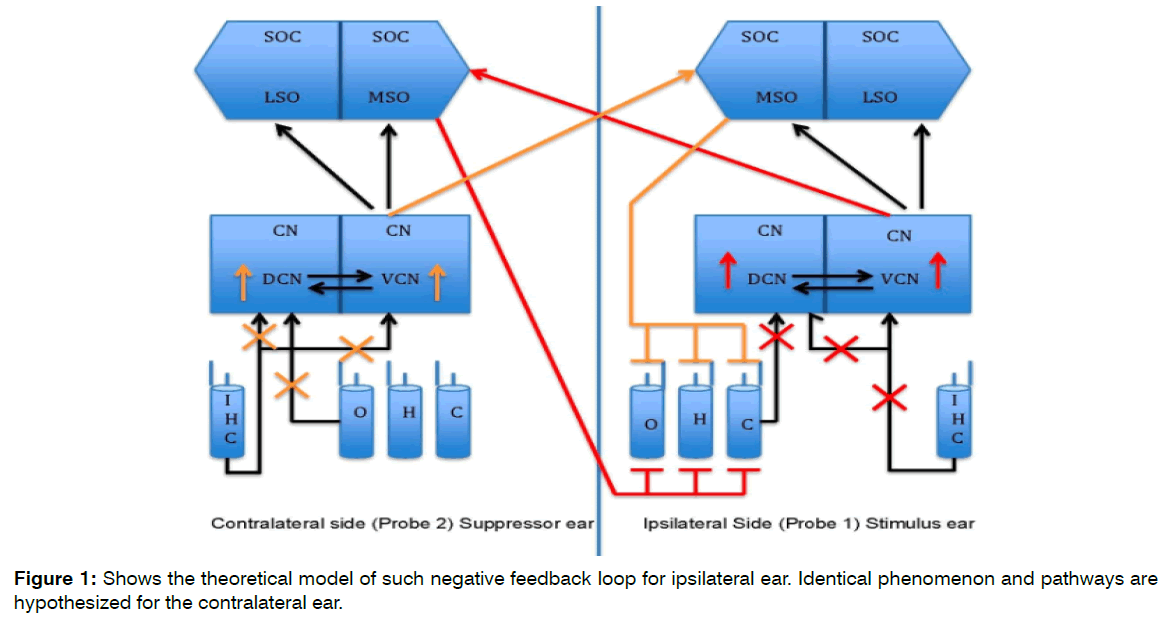
The theoretical model for this study is seen in the Figure 1. Brief duration of silence creates the lack of auditory input. This lack of auditory input/brief duration of sensory deprivation crates the hyperactivity in the cochlear nucleus. Such hyperactivity overexcites ipsilateral and contralateral (shown in red and orange lines) MOC efferent pathway. The overexcitation of MOC creates more suppressive effect on the OHCs activity that in turn should create more auditory input deprivation. This negative feedback loop in turn should create more hyperactivity in the brainstem structures. Figure 1 shows the theoretical model of such negative feedback loop for ipsilateral ear. Identical phenomenon and pathways are hypothesized for the contralateral ear.
Figure 1: Shows the theoretical model of such negative feedback loop for ipsilateral ear. Identical phenomenon and pathways are hypothesized for the contralateral ear.
The suppression of otoacoustic emissions (OAEs) assesses the MOC fibers function. The white noise is presented to the opposite ear (contralateral, non-test ear) and the variation in the OAEs levels is observed in the ipsilateral (test) ear. Suppression of OAEs indicates the inhibitory influence of MOC fibers on cochlear amplification, which in turn reduces the auditory input to the ascending auditory pathway.
Studies in normal hearing individual with tinnitus have reported no suppression effect on Transient Otoacoustic Emissions (TEOAEs) in the tinnitus frequency region, suggestion a MOC fibers dysfunction [12]. This finding is also confirmed by later studies [13,14]. In Tinnitus retraining Therapy (TRT) is an effective tinnitus management program [15] in which patient with tinnitus often asked to avoid silence. Many normal hearing individuals without tinnitus perceive some form of tinnitus after exposure to brief duration of silence in the sound booth [16,17]. Thus, tinnitus has been linked to both silence and MOC dysfunction. The lack of auditory input can trigger or aggravate the perception of tinnitus perhaps via alteration in the function of central auditory neural pathway in which MOC pathway might play a role.
Based on the author’s proposed theoretical model (Figure 1), the purpose of the study is to investigate possible role of the MOC efferent neural pathway and neural connections responsible for tinnitus generation in silence/sensory deprivation. It was hypothesized that:
The total TEOAE amplitude value will be statistically significantly decreased after a period of ten minutes of silence in the test ear (right ear). Rationale: Silence duration would cause the hyperactivity in the cochlear nucleus and would then hyperactivate MOC fibers. Such excess MOC fiber activity would then result in more suppressive effect on the OHCs consequently reducing the total TEOAE amplitude compared to TEOAE amplitude before the silence duration.
The total TEOAE suppression amplitude will be significantly increased after ten minutes of silence in the test ear (right ear). Rationale: Hyperactive MOC fibers would produce more TEOAE suppression and consequently more TEOAE suppression amplitude.
Participants perceiving tinnitus after ten minutes of silence will have greater amount of TEOAE suppression in post-silent measurement than the participants without the perception of tinnitus. Rationale: Since tinnitus has been linked to silence/lack of peripheral auditory input, the participant perceiving tinnitus would have aggressive changes in the brainstem and higher auditory centers than the participants not perceiving tinnitus after the ten minutes of silence. Thus, the greater amount of TEOAE suppression was expected in the tinnitus perceiving participants.
Insert Figure 1 (Color should be used for this figure in print)
Figure 1. Theoretical Model. The cross marks (Red and Orange) show lack of auditory input due to silence in a sound booth. The upward arrows (Red and Orange) in the Cochlear Nucleus (CN) shows hyperactivity due to lack of inhibitory input from the periphery. The Red and Orange pathways shows the inhibitory input to the Outer Hair Cells (OHC) due to hyperactivity in the Cochlear Nucleus (CN). Identical phenomenon and pathways are hypothesized for the contralateral ear.
Materials and Methods
Subjects: Fifty-eight male participants between the age ranges 18-35 years were included in the study. All participants had normal hearing thresholds (< or equal to 25 dB HL in the octave frequencies from 250 Hz to 8000 Hz and at 3000 Hz & 6000 Hz). Each participant had normal middle ear function as evidenced by normal otoscopic examination (No abnormalities or pathologies of the ear canal, including wax) and tympanometry (static compliance between +100 daPa and -100 daPa, 0.33cc middle ear compliance <1.75cc). A case history questionnaire was administered on each participant to collect information on medical history (Appendix A). All participants did not have any history of hearing loss, head trauma, ear surgery, and chronic tinnitus middle ear pathology, prolonged history of noise exposure or trauma, and neurological disease.
Data Collection
This study was approved by the Institutional Review Board (IRB) for the protection of human research participants at the University of North Carolina at Greensboro (IRB number-17-0023). The IRB approved informed consent form was signed by each participant before participating in the study. The recruited participants were instructed to avoid exposure to loud sounds such as music, lawn mowers, motorbikes, gun shots, vacuum cleaners and so forth at least 12 hours before testing. The data was collected in the sound-treated booth meeting ANSI standards.
Instrumentation and Calibration: Auditory hearing sensitivity was assessed using the Grason-Stadler (GSI) 61 clinical audiometer and Eartone 3-A inserts. Middle ear function was assessed using GSI TympStar Middle Ear Analyzer. Otodynamics Echoport ILOV6 292-I instrument was used to measure TEOAEs and contralateral suppression of TEOAEs. All equipment was calibrated at the time of data collection.
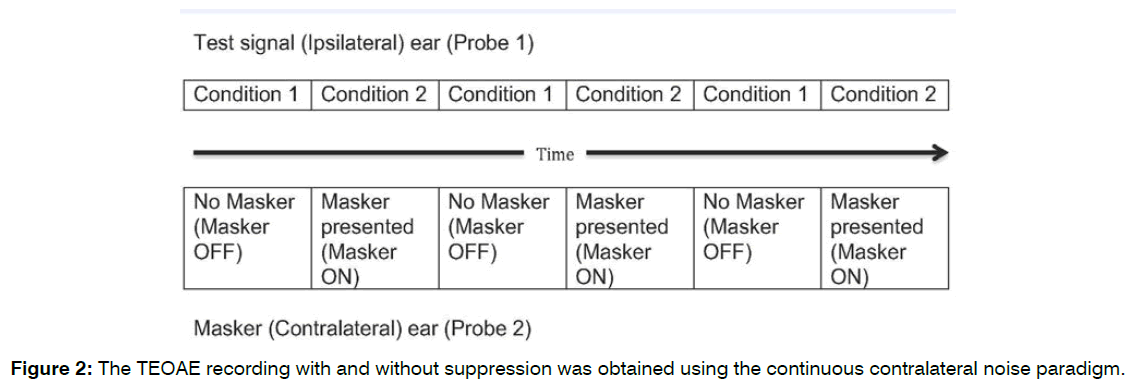
TEOAEs and TEOAEs Suppression Measurement: Continuous Contralateral Noise Paradigm available in the Otodynamics Echoport ILOV6 292-I was used to measure TEOAEs and Contralateral suppression effect of TEOAEs. In Continuous Contralateral Suppressor Noise Method, Probe 1 presented click stimulus in test (Ipsilateral ear). Probe 2 presented suppressor broadband noise in contralateral ear. There were two conditions in this paradigm. In first condition, ‘masker OFF’ condition, the TEOAEs were recorded without suppressor noise. In second condition, ‘masker ON’ condition, TEOAEs were recorded with suppressor noise (Contralateral Suppression of TEOAEs). These two conditions were interleaved for three times to record reliable TEOAEs with and without suppression for better stimulus stability and response reproducibility [18-20]. Three repetitions of each condition were performed with 100 clicks in each condition. The TEOAE and suppression responses were accepted only when the stimulus stability exceeds 80% and the reproducibility of the emissions exceeds 70% [21]. The recommended 60 dB peak sound pressure level click stimulus intensity [21,22]. in linear mode [23] was used. The broadband white noise was used as a contralateral suppressor [18,24]. The suppressor intensity was kept at 65 dB sound pressure level.
Instructions before the Baseline Recording and Silent Period
Tinnitus perception in normal hearing adults changes dur to visual task and auditory attention [25]. In this study, authors observed 68.2% of participant’s perceived tinnitus when seated in the sound booth for 5 minutes during auditory attention task. This percentage reduced to 45.5% when participants were assigned a visual task during the period of silence. Therefore, participant in present study were instructed not to talk, write, or text during the period of silence. Participants were also instructed to disregard the auditory stimulus presented during the OAE tests administered before and after the silent period but report the auditory experience or perception they had during the ten minutes of silent period, if any.
Pre-silence TEOAEs and Contralateral TEOAEs Suppression Recording
Each participant was seated in a comfortable chair inside the sound booth for all TEOAE measurement. Before each testing, OAE probe calibration was completed using the ILO prob-fit check paradigm. The probe (probe 1) was inserted in the right ear canal and the second probe (probe 2) was inserted in the left ear with suitable probe tip. Firm but comfortable seal was obtained in both ears. The click stimulus was delivered to the right ear through probe 1 and the broadband white noise was delivered to the left ear through probe 2. It was ensured that the probe position would not be altered throughout the duration of testing and silence. The TEOAE recording with and without suppression was obtained using the continuous contralateral noise paradigm (Figure 2) and recommended test parameters (mentioned elsewhere). Total TEOAE suppression amplitude was measured by subtracting total TEOAE suppression response from the total TEOAE response.
Figure 2: The TEOAE recording with and without suppression was obtained using the continuous contralateral noise paradigm.
Hood et al. (1996) [21] observed the change in the TEOAE suppression for each of the click intensity from 50, 55, 60, 65, and 70 dB and white noise 10 dB below the click intensity to 10 dB above the click intensity. The variability in the suppression across the subjects was found to be ranged from 0.07 to 0.36 dB with mean of 0.21 dB. The stimulus and suppressor parameters are similar in present study to that of Hood et al. (1996) [21] study. So, 0.36 dB (the upper limit of the range) was considered appropriate to test the suppression effect.
Participant was sitting quietly in a silence/ auditory sensory deprivation condition for 10 minutes.
Post-Silence Teoae and Contralateral Suppression of TEOAEs
To determine any change from the baseline (pre-silence) recording, TEOAE and contralateral suppression of TEOAE were measures again after 10 minutes of silence. It was ensured that the position of the probes remain unaltered throughout the duration of testing and silence. Any difference in the amount of suppression was attributed to the effect of silence/ auditory sensory deprivation.
Questionnaire: Participants were unhooked from the probes and allowed to leave the sound booth. Participant then completed the paper survey (Appendix B) with three questions to indicate the kind of tinnitus perception (such as tone, buzz, cricket-like etc.) that they may have experienced during the silence period. The word ‘Tinnitus’ was intentionally avoided in the tester’s instruction to prevent any apprehension about the auditory perception if any. This step was administered to let the auditory system recover from the changes that silent might induce.
Results
41.4% of the participants perceived some type of tinnitus during/after 10 minutes of silence. Table 1 shows mean, standard deviation, and repeated measure ANOVA of pre and post 10-minute silent total TEOAE and total TEOAE suppression amplitude. No statistically significant difference was found in the total TEOAE amplitude (Wilks’ Lambda=0.984, F (1, 57)=0.948, p=0.334) and TEOAE suppression amplitude (Wilks’s Lambda=0.995, F (1,57)=0.304, p=0.584) before and after 10 minutes of silence.
| 10-minutes silence | M | SD | df | F | Sig. | |
|---|---|---|---|---|---|---|
| Total TEOAE Amplitude | Pre-silence | 11.4897 | 4.9021 | 1 | .948 | .334 |
| Post silence | 11.5828 | 5.0069 | ||||
| Total TEOAE Suppression Amplitude | Pre silence | .9256 | .7470 | 1 | .304 | .584 |
| Post-silence | .8931 | .7525 |
Table 1: Descriptive Statistics and Summary of Repeated Measure ANOVA of TEOAE and TEOAE Suppression Amplitudes: Pre- and Post- 10 Minutes of Silence.
Table 2 shows the mean, standard deviation, and oneway ANOVA of post silent TEOAE suppression between two groups. Post silence total TEOAE suppression between tinnitus perceiving and non-perceiving tinnitus participants was not statistically significantly different (F (1,56)=0.220, ns).
| Post-Silent TEOAE Suppression | |||||||
|---|---|---|---|---|---|---|---|
| N | M | SD | df | F | Sig. | ||
| Tinnitus | Yes | 24 | .8375 | .8303 | 1 | .220 | .641 |
| No | 34 | .9324 | .7026 | ||||
Table 2: Descriptive Statistics and One-Way ANOVA for Post Silence Total TEOAE Suppression Amplitude between Tinnitus Perceiving Participants and Participants not Perceiving Tinnitus.
Table 3 presents the result of repeated measure ANOVA with pre and post silence total TEOAE suppression as the within-subject factors and Tinnitus perception as between subject factor. Tinnitus perception main effect was not statistically significant in TEOAEs (F (1,56)=0.486, p=0.489). The data further shows that there was no statistically significant difference between pre-post 10 minutes silence total TEOAE suppression (PrePostSup) between participants who perceived tinnitus and participants who did not perceive tinnitus during/after 10 minutes of silence (F(1,56)=0.405, p=.527).
| Source | df | F | Sig. |
|---|---|---|---|
| Tinnitus | 1 | .486 | .489 |
| Tinnitus*PrePostSup | 1 | .405 | .527 |
Table 3: Summary of Repeated Measure ANOVA: Main Effect of Tinnitus, Pre- and Post-Silence—Tinnitus Interaction on Total TEOAE Suppression Amplitude.
Discussion
The discussion is based on the three-hypothesis mentioned in the introduction section.
The Effect of Silence on the Teoae Amplitude and Teoae Suppression Amplitude
The result of the study found no statistically significantly difference between total TEOAE amplitude and total TEOAE suppression amplitude before and after ten minutes of silence. To our knowledge, this is the first research study that aims at effect of silence on TEOAE amplitude to investigate MOC efferent pathway. This finding suggests that the brief duration of silence/auditory sensory deprivation does not alter the neural functioning of the lower central auditory neural pathway. It supports the findings [9,26,27] which indicate that the generation of tinnitus is more likely thalamic or higher cortical in origin.
The alternative explanation to such nonsignificant findings could be: A) Ten minutes of silence may not be sufficient to induce hyperactivity in the cochlear nucleus or MOC efferent pathway enough to impose significant TEOAE suppression or may not be sufficient to change the cochlear biomechanics to change the TEOAE amplitude. B) Post silent TEOAE and TEOAE suppression recording might have eliminated any changes in cochlea or hyperactivity in the cochlear nucleus and/or MOC efferent pathway.
Tinnitus Perception and Teoae Amplitude: Present study did not find statistically significant difference in post silent total TEOAE suppression between tinnitus perceiving and non-perceiving participants. There is no interaction effect found between tinnitus and prepost silent TEOAE suppression. Tinnitus perception is considered because of neuroplastic changes in the central auditory system due to some form of cochlear damage [27-29]. It is proposed that the residual peripheral spontaneous activity and central auditory gain collectively contribute to the tinnitus perception [30]. As assessed using audiometric tests and otoacoustic emission tests, participants in the present study did not have any peripheral cochlear damage. This suggests that the pathophysiology of the tinnitus because of cochlear damage might be different than silence induced tinnitus in normal hearing individuals.
Several studies found the MOC efferent pathway dysfunction in the human subjects who had tinnitus but had normal hearing sensitivity [31-34]. The cochlear dead regions that go undetected in the audiometry and even in otoacoustic emissions tests (if such dead regions are outside the test frequency range) can create the hearing loss in frequencies of dead region that in turn can initiate neuroplastic changes in the central auditory system that lead to tinnitus perception. In present study, all participants had normal hearing sensitivity and they did not have tinnitus. After exposing the participants to the ten minutes silence, in participants who did not perceive tinnitus, silence might not have induced the neuroplastic changes in the MOC efferent pathway or cochlear nucleus or higher central auditory system. The ten minutes of silence might have induced temporary neural changes in the central auditory system, but not in MOC efferent or dorsal cochlear nucleus in participant who perceived some form of tinnitus. This also indicates that the silence induced tinnitus might have different pathophysiological mechanism in tinnitus perception than tinnitus associated with hearing loss.
The functional corticofugal efferent system runs from cortex to the cochlea [35]. This system influences lower auditory brainstem structures such as MOC neuronal pathway and cochlear nucleus. Attention plays a significant role in alteration of cerebral cortical area function [36]. Auditory attention influences auditory cortex and other cortical areas [37]. In present study, participants might have sought the sound perception (auditory attention) during the period of silence. Such auditory attention might have suppressed the hyperactivity in the MOC efferent pathway or cochlear nucleus through corticofugal efferent feedback leading to inhibition of significant change in TEOAE suppression after ten minutes of silence.
Limitation of the Study
Being a non-invasive test, contralateral suppression of TEOAE test might not have assessed the altered function of the MOC efferent after a period of silence. The postsilent changes in the MOC efferent recovered soon enough after the stimulus presentation for TEOAE and TEOAE suppression measurement.
Conclusion
The results from this study suggest that the medial olivocochlear efferent pathway or lower brain stem area does not appear to play a role in the emergence of temporary tinnitus in silence but indicate the involvement of higher central auditory nervous system structures in perception of the tinnitus which support the well accepted notion that tinnitus is the central auditory processing phenomenon.
Conflict of Interest
The Authors declares no potential conflict of interest
References
- Møller AR. Tinnitus: presence and future. Progress in Brain Res. 2007;166:3-16.
- American Tinnitus Association. Understanding the facts. (Accessed 2020, at https://www.ata.org/understanding-facts
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hearing Res. 2007;226(1-2):244-53.
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hearing Res. 2009:256(1-2):104-117.
- Cook RD, Hung TY, Miller RL, Smith DW, Tucci DL. Effects of conductive hearing loss on auditory nerve activity in gerbil. Hearing Res. 2002;164(1-2):127-37.
- Pasic TR, Rubel EW. Cochlear nucleus cell size is regulated by auditory nerve electrical activity. Otolaryngol - Head and Neck Surg. 1991;104(1):6-13.
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hearing Res. 2000;147(1-2):26-74.
- Tucci DL, Cant NB, Durham D. Effects of conductive hearing loss on gerbil central auditory system activity in silence. Hearing Res. 2001;155(1-2):124-32.
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neurosci. 2004;27(11):676-82.
- Guinan Jr JJ. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear and Hearing. 2006;27(6):589-607.
- Dhar S, Hall III JW. Otoacoustic emissions: Principles, procedures, and protocols. Plural Publishing; 2018.
- Chéry-Croze S, Collet L, Morgon A. Medial olivo-cochlear system and tinnitus. Acta oto-laryngol. 1993;113(3):285-90.
- Lalaki P, Hatzopoulos S, Lorito G, Kochanek K, Sliwa L, Skarzynski H. A connection between the Efferent Auditory System and Noise-Induced Tinnitus Generation. Reduced contralateral suppression of TEOAEs in patients with noise-induced tinnitus. Medical science monitor: Int Med J Exp and Clin Res. 2011;17(7):MT56.
- Paglialonga A, Del Bo L, Ravazzani P, Tognola G. Quantitative analysis of cochlear active mechanisms in tinnitus subjects with normal hearing sensitivity: Multiparametric recording of evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010;37(3):291-8.
- Jastreboff PJ. Tinnitus habituation therapy (THI) and tinnitus retraining therapy (THI). Tinnitus Handbook. 2000:357-76.
- Heller MF, Bergman M. VII Tinnitus aurium in normally hearing persons. Ann of Otol, Rhinol & Laryngol. 1953;62(1):73-83.
- Tucker DA, Phillips SL, Ruth RA, Clayton WA, Royster E, Todd AD. The effect of silence on tinnitus perception. Otolaryngol—Head and Neck Surg. 2005;132(1):20-4.
- Berlin CI, Hood LJ, Wen H, Szabo P, Cecola RP, Rigby P, et al. Contralateral suppression of non-linear click-evoked otoacoustic emissions. Hearing Res. 1993;71(1-2):1-1.
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hearing Res. 1990;43(2-3):251-61.
- Ryan S, Kemp DT, Hinchcliffe R. The influence of contralateral acoustic stimulation on click-evoked otoacoustic emissions in humans. British J Audiol. 1991;25(6):391-7.
- Hood LJ, Berlin CI, Hurley A, Cecola RP, Bell B. Contralateral suppression of transient-evoked otoacoustic emissions in humans: intensity effects. Hearing Res. 1991;101(1-2):113-8.
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol. 1991;65(3):724-35.
- Hood LJ. Suppression of otoacoustic emissions in normal individuals and in patients with auditory disorders. Otoacoustic Emissions: Clinical Applications. New York: Thieme Med Publishers. 2002:325-47.
- Velenovsky DS, Glattke TJ. The effect of noise bandwidth on the contralateral suppression of transient evoked otoacoustic emissions. Hearing Res. 2002;164(1-2):39-48.
- Knobel KA, Sanchez TG. Influence of silence and attention on tinnitus perception. Otolaryngol—Head and Neck Surg. 2008;138(1):18-22.
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hearing Res. 2000;142(1-2):89-101.
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neurosci. 2004;27(11):676-82.
- Kaltenbach JA. Tinnitus: Models and mechanisms. Hearing Res. 2011;276(1-2):52-60.
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30(45):14972-9.
- Noreña AJ, Farley BJ. Tinnitus-related neural activity: Theories of generation, propagation, and centralization. Hearing Res. 2013;295:161-71.
- Fernandes LD, Santos TM. Tinnitus and normal hearing: a study on the transient otoacoustic emissions suppression. Brazilian J Otorhinolaryngol. 2009;75(3):414-9.
- Granjeiro RC, Kehrle HM, Bezerra RL, Almeida VF, André LS, Oliveira CA. Transient and distortion product evoked oto-acoustic emissions in normal hearing patients with and without tinnitus. Otolaryngolo—Head and Neck Surg. 2008;138(4):502-6.
- Paglialonga A, Del Bo L, Ravazzani P, Tognola G. Quantitative analysis of cochlear active mechanisms in tinnitus subjects with normal hearing sensitivity: multiparametric recording of evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010;37(3):291-8.
- Riga M, Papadas T, Werner JA, Dalchow CV. A clinical study of the efferent auditory system in patients with normal hearing who have acute tinnitus. Otol & Neurotol. 2007;28(2):185-90.
- Perrot X, Ryvlin P, Isnard J, GuÈnot M, Catenoix H, Fischer C, et al. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cerebral Cortex. 2006;16(7):941-8.
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54(5):677-96.
- Jastreboff PJ. The neurophysiological model of tinnitus and hyperacusis. InProceedings of the sixth international tinnitus seminar. The Tinnitus and Hyperacusis Centre Cambridge UK. 1999:32-38.
1Department of Communication Sciences and Disorders, Edinboro University of Pennsylvania, Human Services Building Room 237, 215 Scotland Rd, Edinboro, USA
2Department of Communication Sciences and Disorders, University of North Carolina Greensboro, 317 Ferguson Building, Greensboro, USA
Send correspondence to:
Amitkumar Tayade
Department of Communication Sciences and Disorders, Edinboro University of Pennsylvania, 215 Scotland Road, Room 237, Edinboro, PA 16444, USA, E-mail: atayade@pennwest.edu, amittayade11@gmail.com
Phone: (336) 681-0047.
Paper submitted on February 16, 2022; and Accepted on March 14, 2022
Citation: Amitkumar Tayade, Denise Tucker. Evaluation of Medial Olivocochlear Neural Efferent Pathway in Tinnitus Perception in Normal-hearing Individuals. Int Tinnitus J. 2022;26(1):20-26.