The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 28, Issue 2 / December 2024
Research Article Pages:177-186
10.5935/0946-5448.20240026
Evaluation of Osteoporosis in Postmenopausal Women by Dental Panoramic Radiographic Indices as a Predictor of Osteoporosis and Relation to Some Serum Biochemical Markers; A Case Control Study
Authors: Khoshee Salih Hameed, Asma Qasim Rahman, Shaheen Ali Ahmed, Kharman Khidhr Rahman, Ali Fakhree Al Zubaidee
PDF
Abstract
Background: Osteoporosis is a bone metabolic disease, characterized by decreased bone mass density and impaired micro-architecture of bone tissue, is seen commonly with increasing age. Mandibular inferior cortical shape and width on dental panoramic radiographs may be useful screening tools for osteoporosis. The aim of this study was to assess mandibular bone mineral density by using panoramic radio morphometric indices and determine the relation between theses indices with some serum biochemical markers. Materials and Methods: This study was enrolled forty-three post-menopausal women as a case group and thirty pre-menopausal women as a control group. Quantitative mandibular radio-morphometric indices include the Mandibular Cortical Index (MCI), the Mental Index (MI) and Panoramic Mandibular Index (PMI) which were measured on digital panoramic radiographs, and serum, calcium, phosphate, vitamin D, estradiol and parathyroid hormone levels were evaluated. Result: Two groups, 43 menopausal women (cases), and 43 premenopausal women (control). Around one third (32.6)) of cases have established osteoporosis (C3 category of MCI), compared with 0% of the control group (p < 0.001) which is highly statistically significant difference between the 2 groups, other panoramic indices do not show any statistically significant differences between the 2 groups, the mean of estradiol level among women of C3 category (5.42) is very low, compared with 70.88 among C1 women and 59.43 among C2 women (p < 0.001). other parameters do not show any significant differences between the 2 groups. Low levels of MI, IPMI, and SPMI is found among women of C3 MCI category, and all the differences between the MCI categories were significant (p= 0.021, p= 0.003, and p= 0.024 respectively Conclusion: The mandibular cortical index, and shape on the dental panoramic radiographs may be used as an indicator of bone turnover. In this study there is a correlation between estradiol and osteoporosis.
Keywords: Mandibular Cortical Index, Mental Index, Menopause, Osteoporosis, Panoramic Mandibular Index
Keywords
Mandibular Cortical Index, Mental Index, Menopause, Osteoporosis, Panoramic Mandibular Index.
Introduction
Bone Mineral Density (BMD) refers to the quantity of bone tissue contained inside a specific volume of bone [1]. Osteoporosis is a condition that affects the bones. It is characterized by a decrease in bone mass and an alteration in the structure of bone tissue, resulting in bones being more fragile and susceptible to fractures [2]. Evaluating jaws (BMD) might be deemed valuable, or even essential, in various clinical scenarios, including oral and/or systemic illnesses, implant preparation, therapy assessment, and post-treatment monitoring [3]. Smoking, alcohol consumption, physical inactivity, genetics, poor calcium intake, pharmacological therapy with glucocorticoids, antiepileptic and anticoagulant drugs, and disorders that alter bone metabolism are all risk factors for osteoporosis [4]. The primary factor contributing to reduced bone mass in women is the hormonal fluctuations associated with menopause [5]. However, there are other factors that also affect bone mineral density, such as bone metabolism, skeletal mineral status, tooth extraction, surgical interventions, occlusal forces exerted by dentures, physical and muscular activity, presence of the remaining teeth, denture support, mandibular bone thickness, and body mass index [6]. Detecting asymptomatic persons who are susceptible to fractures is crucial for managing the rise in morbidity, mortality, and healthcare expenses on a global scale [7].
Dental radiographs, particularly panoramic pictures, have been utilized to anticipate patients with low (BMD) [8-10]. The majority of the researches have primarily examined the thickness and structural integrity of the lower border of the mandible. The mandibular radio-morphometric indices consist of the Mandibular Cortical Index (MCI), the Mental Index (MI) [11], and the Panoramic Mandibular Index (PMI) [12]. The panoramic indices are crucial as they are used every day by dentists to evaluate the state of the mandible. It could be used to assess the level of bone mineralization, particularly in high-risk individuals such as post-menopausal women, as a straight forward and noninvasive method [13].A study conducted in the United States indicated that there is a correlation between mandibular cortical measurements on dental panoramic radiographs and a higher likelihood of experiencing an osteoporotic fracture [14].
During the menopausal transition, the production of estrogen and progesterone by the ovaries decreases. The normal hormonal shift is linked to reduced levels of estradiol (E2) in the blood [15]. The cessation of estrogen during menopause may render bones more susceptible to oxidative damage, hence increasing the likelihood of developing postmenopausal osteoporosis [16].The primary cause of bone loss in postmenopausal women is estrogen insufficiency, which disrupts the equilibrium between osteoclast resorption and bone production regulated by osteoblasts [17]. Several studies have examined the relationship between estradiol indicators and bone mineral density in postmenopausal women, either directly or indirectly [18-20].
Calcium and phosphorous are metallic elements that exist as deposits within the bone. Calcium ranks as the fifth most prevalent mineral in the body [21]. Calcium mineral has two primary functions in the body: maintaining structural integrity and regulating metabolic activity [22]. Vitamin D primarily functions as a hormone, and its endocrine activity regulates the balance of blood calcium and phosphate by controlling its absorption in the intestines [23-25]. Primary hyperparathyroidism (PHPT) is a calcium metabolic condition that is most commonly observed in postmenopausal women [26]
Multiple investigations have demonstrated a reduction of (BMD) in individuals with (PHPT) [27]. Parathyroid hormone (PTH) typically serves as the primary controller of calcium balance and primarily affects the kidneys and bones [28]. It enhances the reabsorption of calcium in kidney cells and promotes the conversion of 25-hydroxy vitamin D to 1,25-dihydroxyvitamin D via activating 1,25-hydroxylase [29]. In PHPT, there is an excessive production of PTH, which results in higher amounts of calcium in the bloodstream as a result of its release from the bone reservoirs. Studies have demonstrated that this leads to an elevated susceptibility to osteoporosis due to the accelerated pace of bone remodeling [28].
The objective of this study is to assess the density of the mandibular bone by utilizing panoramic radio-morphometric indices. Additionally, to establish the correlation between these indices and the levels of serum calcium, phosphate, vitamin D, estradiol, and parathyroid hormone.
Patients and Methods
This investigation was conducted as a prospective case-control clinical study at the private NADWA polyclinic The research protocol was approved by Ethical Committee of College of Dentistry Hawler Medical University. Prior to the study, all subjects involved in this study were provided with and signed informed consent forms. There was no medical treatment provided and the patients' information was kept private [29].
Sample size determination: The selected sample was included 43 post-menopausal women, (47-76) year of age (55.906±1.16 years) as the case group , who do not have any menstruation for at least 1 year , and 30 pre-menopausal women below 43 years of age (32.63±1.2 years as the control group. In both groups, patients visited the radiology department to undergo Orthopantomography (OPG) for dental reasons. Demographic information of the patient includes age, smoking status, and menopausal status.
Exclusion criteria
1. Patients with a medical history of severe conditions impacting the bones in the mouth, endocrine system, metabolism, or skeletal system, as well as women undergoing hormone replacement therapy or using medications such as calcitonin, bisphosphonates, calcium, and vitamin D.
2. Subjects who were smokers or alcoholic or had a recent fracture (within 2 years) or osseous lesions in the mandibles.
Blood Sampling
Under standardized settings, venous blood samples were extracted from each participant. Within 30 minutes, blood samples were prepared and stored at a temperature of -80°C. These samples were then analyzed for serum biomarkers. The levels of (E2) and (PTH) were determined using commercially available immunoassays. The measurement of serum 25-hydroxy-vitamin D (25[OH] D) was performed using a direct competitive chemiluminescence immunoassay on the LIASON autoanalyzer. Additionally, the levels of serum calcium and phosphate were also assessed
Panoramic indices measurements
All panoramic views were captured using the Vatech digital X-ray equipment, model HDG-07BT2.13, manufactured by Samsung at their facility located in 1ro2 gilHwaswong-si, Gyeonggi-do 18449, KOREA in 2018. Using a single operator, the task was performed at 12 mega-amperes, 15 seconds, and 70-80 kilovolts. The position of the head was maximally standardized. Linear measurements were conducted by employing a digital calliper and overlaying a transparent plastic acetate sheet on panoramic radiographs, following a 20% magnification adjustment to more accurately replicate the clinical scenario.
1. MCI is divided into three groups based on the criteria outlined by Klemetti et al [30]. C1: The endosteal margin of the cortex is uniformly smooth and distinct on both sides. C2: The endosteal margin exhibits crescent-shaped defects (lacunar resorption) and/or appears to create residual cortical material on one or both sides. C3: The cortical layer produces substantial residual cortical material along the endosteal margin and displays evident porosity (Figure 1).
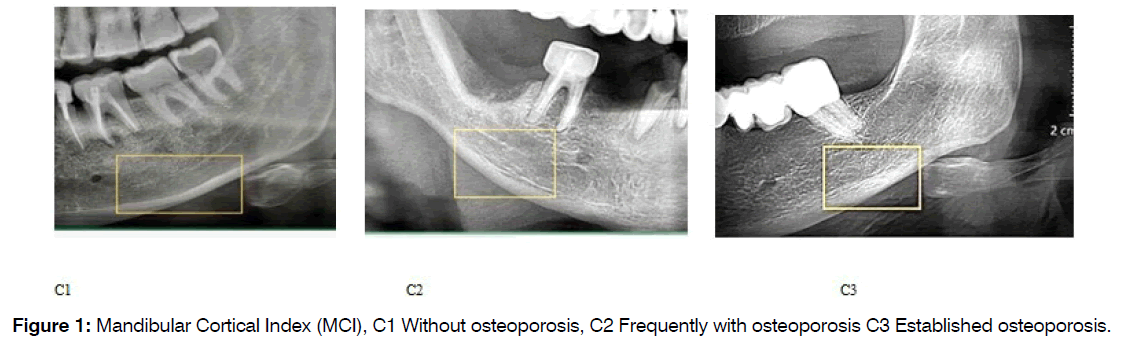
Figure 1: Mandibular Cortical Index (MCI), C1 Without osteoporosis, C2 Frequently with osteoporosis C3 Established osteoporosis.
2. (MI): Mental Index was measured on the line which was perpendicular to the bottom of the mandible at the middle of the mental foramen (8) (normal value > 3.1mm).
3. (PMI): base on the criteria outlined by in 1991 by Benson et al. The PMI is the ratio of the thickness of the mandibular cortex to the distance between the mental foramen and the inferior mandibular cortex (Normal value > 0.3) Panoramic Mandibular Index (PMI) [31]. Ratio of b/a Superior and inferior PMI were calculated as:
Superior PMI (SPMI)= Cortical thickness(C)/distance from superior margin of mental foramen to inferior border of mandible(S)
Inferior PMI (IPMI)= Cortical thickness(C)/distance from inferior margin of mental foramen to inferior border of mandible(I) (Figure 2) [32].
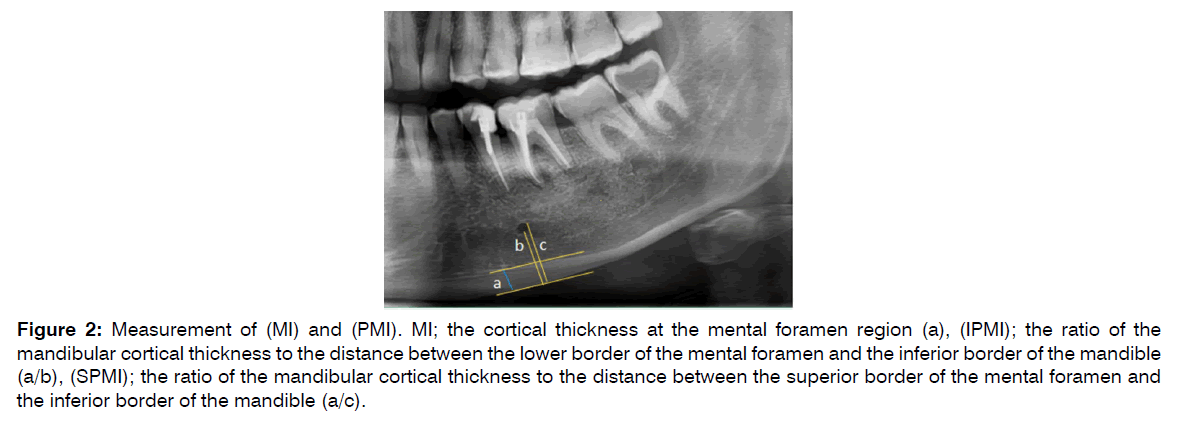
Figure 2: Measurement of (MI) and (PMI). MI; the cortical thickness at the mental foramen region (a), (IPMI); the ratio of the mandibular cortical thickness to the distance between the lower border of the mental foramen and the inferior border of the mandible (a/b), (SPMI); the ratio of the mandibular cortical thickness to the distance between the superior border of the mental foramen and the inferior border of the mandible (a/c).
Intra- and inter-observer agreement: To quantify intra-observer agreements, 50 panoramic radiographs was randomly selected from the sample studied and examined twice by the main observer. There was a period of 4 weeks between readings. Another observer was also analyzed the same radiographs to verify interobserver reliability.
A paired 2-tailed t-test was used to assess the difference in measurements between the left and right sides of the mandible, together with the intra- and inter-observer reading which result no statistically no signific different between them P-value =1.00.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS, version 26). Normality of data was checked using the Shapiro-Wilk test, accordingly non-parametric tests were used when indicated. Chi square test of association was used to compare proportions. Fisher’s exact test was used (instead of the Chi square test) when the expected frequency (value) was less than 5 of more than 20% of the cells of the table. Spearman rho correlation coefficient was calculated to assess the strength of correlations between variables. Kruskal-Wallis test was used to compare the mean ranks of three groups, and a post-hoc test (LSD) was used to compare each two groups. A p value of ≤ 0.05 was considered as statistically significant.
Results
Two groups of women were included in the study, 43 menopausal women (cases), and 43 premenopausal women (control). Around one third (32.6%) of cases had established osteoporosis (C3 category of MCI), compared with 0% of the control group (p < 0.001) (Table 1).
| Cases (Menopause) | Control | Total | ||
|---|---|---|---|---|
| Mandibular Cortical Index | No. (%) | No. (%) | No. (%) | P value* |
| C1.Without osteoporosis | 7 (16.3) | 18 (41.9) | 25 (29.0) | < 0.001 |
| C2.Frequently with osteoporosis | 22 (51.2) | 25 (58.1) | 47 (54.7) | |
| C3.Established osteoporosis | 14 (32.6) | 0 (0.0) | 14 (16.3) | |
| Total | 43 (100.0) | 43 (100.0) | 86 (100.0) |
Table 1: Mandibular Cortical Index (MCI) categories by menopausal status
More than half (52.3%) of the studied sample had low MI, but there was no significant difference between the two groups (p = 0.829). Regarding the IPMI, 48.8% of the whole sample had low IPMI, but the difference was not significant between the groups (p = 0.084). The majority (89.5%) of the women had low SPMI, again the difference was not significant (p > 0.999), as presented in (Table 2).
| Case | Control | Total | ||
|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | p | |
| Mental index (MI) | ||||
| Normal | 20 (46.5) | 21 (48.8) | 41 (47.7) | |
| Low | 23 (53.5) | 22 (51.2) | 45 (52.3) | 0.829* |
| Inferior Panoramic Mandibular Index (IPMI) | ||||
| Normal | 18 (41.9) | 26 (60.5) | 44 (51.2) | |
| Low | 25 (58.1) | 17 (39.5) | 42 (48.8) | 0.084* |
| Superior Panoramic Mandibular Index (SPMI) | ||||
| Normal | 5 (11.6) | 4 (9.3) | 9 (10.5) | |
| Low | 38 (88.4) | 39 (90.7) | 77 (89.5) | >0.999** |
| Total | 43 (100.0) | 43 (100.0) | 86 (100.0) | |
Table 2: Mandibular Cortical Index (MCI) categories by menopausal status
Considering the menopausal women only (cases), no significant association was detected between the mental index (MI) and the MCI categories (p = 0.070). Regarding the IPMI, the majority (85.7%) of the C3 women had low IPMI, compared with 45.5% of C2 women and 42.9% of C1 women (p = 0.044). No significant association was detected between the SPMI and the MCI categories (p = 0.545).
Considering the premenopausal women only, no significant association was detected between mental index (MI) and the MCI categories (p = 0.455). Around half (52%) of C2 women had low IPMI, compared with 22.2% of C1 women (p = 0.049). No significant association was detected between SPMI and the MCI categories in the premenopausal women (p = 0.628), as presented in (Table 3).
| MCI† | |||||
|---|---|---|---|---|---|
| C1 | C2 | C3 | |||
| No. (%) | No. (%) | No. (%) | No. (%) | p | |
| Cases | |||||
| MI | |||||
| Normal | 4 (57.1) | 13 (59.1) | 3 (21.4) | 20 (46.5) | |
| Low | 3 (42.9) | 9 (40.9) | 11 (78.6) | 23 (53.5) | 0.070** |
| IPMI | |||||
| Normal | 4 (57.1) | 12 (54.5) | 2 (14.3) | 18 (41.9) | |
| Low | 3 (42.9) | 10 (45.5) | 12 (85.7) | 25 (58.1) | 0.044** |
| SPMI | |||||
| Normal | 0 (0.0) | 4 (18.2) | 1 (7.1) | 5 (11.6) | |
| Low | 7 (100.0) | 18 (81.8) | 13 (92.9) | 38 (88.4) | 0.545** |
| Total | 7 (100.0) | 22 (100.0) | 14 (100.0) | 43 (100.0) | |
| Controls | |||||
| MI | |||||
| Normal | 10 (55.6) | 11 (44.0) | 0 (0.0) | 21 (48.8) | |
| Low | 8 (44.4) | 14 (56.0) | 0 (0.0) | 22 (51.2) | 0.455* |
| IPMI | |||||
| Normal | 14 (77.8) | 12 (48.0) | 0 (0.0) | 26 (60.5) | |
| Low | 4 (22.2) | 13 (52.0) | 0 (0.0) | 17 (39.5) | 0.049* |
| SPMI | |||||
| Normal | 1 (5.6) | 3 (12.0) | 0 (0.0) | 4 (9.3) | |
| Low | 17 (94.4) | 22 (88.0) | 0 (0.0) | 39 (90.7) | 0.628** |
| 18 (100.0) | 25 (100.0) | 0 (0.0) | 43 (100.0) | ||
Table 3: Mandibular Cortical Index (MCI) categories by menopausal status
C1. Without osteoporosis. C2. Frequently with osteoporosis. C3. Established osteoporosis.
It is evident in (Table 4) that higher means and means ranks of age are significantly associated with higher degree (rank) of MCI (p < 0.001). No significant (p = 0.666) difference was detected between the three categories of MCI regarding Calcium (Ca),but it is clear that the mean of Estradiol level among women of C3 category (5.42) was very low, compared with 70.88 among C1 women and 59.43 among C2 women (p < 0.001). No significant differences were detected between the MCI categories regarding phosphate (p = 0.110), D3 (p = 0.574), and PTH (p = 0.722). Low levels of MI, IPMI, and SPMI were found among women of C3 MCI category, and all the differences between the MCI categories were significant (p = 0.021, p = 0.003, and p = 0.024 respectively). More details are presented in (Table 4).
| MCI | Mean | SD | Mean Rank | P* | Groups | P** | |
|---|---|---|---|---|---|---|---|
| Age | C1 | 36.16 | 11.02 | 31.48 | C1XC2 | 0.128 | |
| C2 | 41.53 | 13.76 | 40.89 | < 0.001 | C1XC3 | < 0.001 | |
| C3 | 61 | 8.57 | 73.71 | C2XC3 | < 0.001 | ||
| Total | 43.14 | 14.71 | |||||
| Ca | C1 | 9.95 | 0.8 | 40.36 | C1XC2 | N/A | |
| C2 | 10.17 | 0.69 | 45.67 | 0.666 | C1XC3 | N/A | |
| C3 | 10.07 | 0.49 | 41.82 | C2XC3 | N/A | ||
| Total | 10.09 | 0.7 | |||||
| Estradiol | C1 | 70.88 | 43.7 | 54.74 | C1XC2 | 0.124 | |
| C2 | 59.43 | 61.31 | 45.22 | < 0.001 | C1XC3 | < 0.001 | |
| C3 | 5.42 | 4.43 | 17.64 | C2XC3 | 0.001 | ||
| Total | 53.97 | 55.37 | |||||
| Phosphate | C1 | 3.45 | 0.56 | 34.68 | C1XC2 | N/A | |
| C2 | 3.71 | 0.44 | 46.89 | 0.11 | C1XC3 | N/A | |
| C3 | 3.75 | 0.73 | 47.86 | C2XC3 | N/A | ||
| Total | 3.64 | 0.54 | |||||
| D3 | C1 | 19.89 | 14.2 | 41.12 | C1XC2 | N/A | |
| C2 | 21.02 | 12.89 | 42.93 | 0.574 | C1XC3 | N/A | |
| C3 | 27.5 | 19.56 | 49.68 | C2XC3 | N/A | ||
| Total | 21.75 | 14.56 | |||||
| PTH | C1 | 48.63 | 27.15 | 42.66 | C1XC2 | N/A | |
| C2 | 45.34 | 20.23 | 42.48 | 0.722 | C1XC3 | N/A | |
| C3 | 53.25 | 23.66 | 48.43 | C2XC3 | N/A | ||
| Total | 47.58 | 22.88 | |||||
| MI | C1 | 3.33 | 0.42 | 52.82 | C1XC2 | 0.098 | |
| C2 | 3.18 | 0.65 | 42.6 | 0.021 | C1XC3 | 0.006 | |
| C3 | 2.85 | 0.59 | 29.89 | C2XC3 | 0.095 | ||
| Total | 3.17 | 0.6 | |||||
| IPMI | C1 | 0.33 | 0.05 | 51.88 | C1XC2 | 0.251 | |
| C2 | 0.32 | 0.08 | 44.8 | 0.003 | C1XC3 | 0.001 | |
| C3 | 0.26 | 0.05 | 24.18 | C2XC3 | 0.007 | ||
| Total | 0.31 | 0.07 | |||||
| SPMI | C1 | 0.25 | 0.04 | 51.66 | C1XC2 | 0.185 | |
| C2 | 0.24 | 0.06 | 43.49 | 0.024 | C1XC3 | 0.006 | |
| C3 | 0.21 | 0.04 | 28.96 | C2XC3 | 0.055 | ||
| Total | 0.24 | 0.05 |
N/A: Not applicable (Post-hoc p values were not calculated by the SPSS as the p values of the Kruskal-Wallis test were not significant).
Table 4: Means and mean ranks of numerical variables by Mandibular Cortical Index (MCI) categories
Considering the menopausal women (cases) only, all the correlations between age and the variables mentioned in (Table 5) were not significant. Positive significant correlation was found between calcium and phosphate albeit it was not strong (rho = 0.381, p = 0.012). The other significant correlations were between Estradiol and phosphate (rho = 0.329, p = 0.031), D3 and PTH (rho = -0.316, p = 0.039), and D3 and MI (rho = 0.387, p = 0.010). Stronger correlations were found between MI and IPMI (rho = 0.657, p < 0.001), MI and SPMI (rho = 0.773, p < 0.001). Also, between IPMI and SPMI (rho = 0.907, p < 0.001). The other correlations were not significant (Table 5).
| Ca | Estradiol | Phos-phate | D3 | PTH | MI | IPMI | SPMI | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | rho | -0.153 | -0.189 | -0.151 | -0.104 | 0.189 | -0.018 | -0.078 | -0.051 |
| p | 0.328 | 0.224 | 0.334 | 0.507 | 0.224 | 0.91 | 0.617 | 0.744 | |
| Ca | rho | 0.277 | 0.381 | 0.229 | -0.125 | 0.236 | 0.076 | 0.219 | |
| p | 0.072 | 0.012 | 0.139 | 0.423 | 0.128 | 0.626 | 0.158 | ||
| Estradiol | rho | 0.329 | 0.054 | -0.174 | 0.026 | 0.046 | 0.069 | ||
| p | 0.031 | 0.73 | 0.265 | 0.871 | 0.769 | 0.659 | |||
| Phosphate | rho | 0.154 | -0.192 | -0.189 | -0.033 | -0.032 | |||
| p | 0.324 | 0.216 | 0.224 | 0.834 | 0.839 | ||||
| D3 | rho | -0.316 | 0.387 | 0.108 | 0.202 | ||||
| p | 0.039 | 0.01 | 0.492 | 0.193 | |||||
| PTH | rho | -0.141 | -0.068 | -0.071 | |||||
| p | 0.368 | 0.666 | 0.652 | ||||||
| MI | rho | 0.657 | 0.773 | ||||||
| p | < 0.001 | < 0.001 | |||||||
| IPMI | rho | 0.907 | |||||||
| p | < 0.001 |
Table 5: Correlations between the numerical variables among the cases
Considering the premenopausal women (control) only, the significant correlations were as follows: age and Estradiol (rho = -0.414, p = 0.006); Ca and D3 (rho = 0.327, p = 0.032); D3 and PTH (rho = -0.339, p = 0.026); MI and IPMI (rho = 0.704, p < 0.001); MI and SPMI (rho = 0.822, p < 0.001); IPMI and SPMI (rho = 0.883, p < 0.001). The other correlations mentioned in (Table 6) were not significant.
| Ca | Estradiol | Phos-phate | D3 | PTH | MI | IPMI | SPMI | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | rho | 0.124 | -0.414 | 0.002 | 0.316 | 0.2 | -0.158 | -0.298 | -0.208 |
| p | 0.427 | 0.006 | 0.989 | 0.039 | 0.198 | 0.311 | 0.052 | 0.18 | |
| Ca | rho | 0.053 | 0.036 | 0.327 | -0.254 | -0.064 | 0.01 | 0.104 | |
| p | 0.734 | 0.817 | 0.032 | 0.1 | 0.682 | 0.949 | 0.509 | ||
| Estradiol | rho | 0.067 | -0.153 | -0.154 | 0.244 | 0.106 | 0.148 | ||
| p | 0.668 | 0.327 | 0.323 | 0.114 | 0.499 | 0.342 | |||
| Phosphate | rho | 0.295 | -0.306 | -0.152 | -0.212 | -0.207 | |||
| p | 0.055 | 0.046 | 0.332 | 0.172 | 0.182 | ||||
| D3 | rho | -0.339 | -0.22 | -0.15 | -0.183 | ||||
| p | 0.026 | 0.156 | 0.337 | 0.241 | |||||
| PTH | rho | -0.205 | -0.202 | -0.288 | |||||
| p | 0.187 | 0.194 | 0.061 | ||||||
| MI | rho | 0.704 | 0.822 | ||||||
| p | < 0.001 | < 0.001 | |||||||
| IPMI | rho | 0.883 | |||||||
| p | < 0.001 |
Table 6: Correlations between the numerical variables among the control group
Discussion
Dental panoramic radiography is commonly used to diagnose illnesses that impact the dento-maxillofacial complex. Dental panoramic radiographs can be utilized to identify women who are unaware of their low (BMD) and might benefit from BMD testing [33]. These radiographs may reveal accidental findings that can serve as indicators for further evaluation.
The objective of this screening is not to provide a diagnosis of osteoporosis, but rather to identify persons who are at risk of developing osteoporosis and send them to the proper medical care. The MCI serves as a qualitative measure of cortical morphology. Research findings indicate that utilizing panoramic radiographs for MCI classification could serve as a valuable diagnostic tool for identifying osteoporosis. Research has demonstrated a substantial correlation between the average (BMD) of the lower jaw, as measured by dual-energy X-ray absorptiometry (DXA), and the MCI scale. Specifically, mandibles classed as C3 exhibit the lowest BMD [34]. Furthermore, clinical dentists play a crucial role in identifying mandibular cortical erosion observed in panoramic radiographs of postmenopausal women. This study is the first of its kind to focus on Kurdistanion women living in Erbil, specifically examining the impact of menopausal status on mandibular cortical index (MCI) and osteoporosis. The statistical analysis showed a highly significant difference between the two groups regarding MCI, which aligns with findings from other studies, such as Mudda et al [35]. These results suggest that the menopausal status of patients is associated with changes in mandibular cortical morphology. The prevalence of MC1 was highest among the pre-menopausal population.
The presence of MCI3 phenotype was identified exclusively in the post-menopausal group, occurring only beyond the age of 54 years. Additionally, there was a noticeable increase in the number of patients displaying the C3 phenotype as they aged, indicating age-related alterations. The present investigation concurs with the findings of Knezovic-Zlataric et al [36], manifesting alterations associated with the ageing process. Therefore, our study found a correlation between the (MCI) and menopausal state.
Significant focus has been given to the screening of low skeletal bone mass screening based on cortical width measurements at the mental foramen area [37]. Dutra et al [38] demonstrated the accuracy of measuring MI in panoramic radiographs, which effectively reflects the actual bone condition. The results of (MI) shown a negative correlation with age, meaning that the mean values decreased as age increased. In our investigation, we found that there was no statistically significant difference in MI values between the pre-menopausal group and the post-menopausal group. This discrepancy arises from the previous study, by Mudda et al which reported that the mean MI was 4.99 in the pre-menopausal group and 4.46 in the postmenopausal group (P<0.05). Our studyis the first known research conducted on Kurdish women,
The PMI, or radio morphometric approach, was introduced in 1991 by Benson et al [11]. It has been proposed that although there is bone loss in the alveolar region above the foramen, the distance between the foramen and the lower edge of the mandible remains relatively constant throughout life. Also, the selection of a ratio as the basis of the PMI represented an attempt which was made to compensate for image distortion and magnification which were inherent in panoramic imaging Hence, the PMI serves as a quantification of the thickness of the mandibular cortex in relation to its normal size, making it a valuable tool for assessing localized bone loss in dental treatment [32].
Our study found no statistically significant differences in the mean values of PMI between the pre-menopausal group and the post-menopausal group. This contradicts the findings of the study by Mudda et al [35], which reported higher mean values in the pre-menopausal group compared to the post-menopausal group, with statistically significant differences between the two groups. The findings align with those of Taguchi et al, [33], who proposed that in females, PMI exhibits a progressive rise until the sixth decade and then declines. This pattern was observed in our study, which included a small number of post-menopausal females aged beyond 60 years. The study conducted by Balto et al [39] found that the values of PMI were not significant, suggesting that they had little to no usefulness in detecting or diagnosing osteoporosis in postmenopausal females.
The mean of IPMI in the established osteoporotic group (MCI3) was found to be lower than (MCI2) and (MCI1) groups. These differences were highly significant. The mean of MI in group (MCI3) was lower than in (MCI2) and (MCI1). The observed differences in our study regarding the relationship between PMI, MI, and different types of MCI were found to be statistically significant. Previous studies did not investigate this specific relationship. Our study revealed a positive correlation between IPMI and MI with MCI3. However, other studies have found relation between PMI and MI with osteoporosis of the mandible, as determined by DXA scan. These studies also reported a statistically significant difference in mean PMIs between the DEXA groups (p = 0.007). This suggests a correlation between low bone mass density in post-menopausal women and PMI, as reported by Gaur et al [40].
Postmenopausal bone loss can be divided into two phases. The first phase is characterised by a rapid decline in bone mass, which is dependent on estrogen levels. The second phase is an age-related bone loss that occurs at a constant, slower rate throughout the ageing process. This study found that the average level of estradiol, a type of estrogen, in postmenopausal women was lower than that in premenopausal women. This difference was statistically significant, indicating a strong association. Additionally, the study found that the average level of serum estradiol was lower in the group with (MCI3) compared to the groups with (MCI2) and (MCI1), these differences were also statistically significant. The findings of this study were consistent with those of Reddy Kilim and Chandala. In their study, the average levels of estradiol in premenopausal women were 175.48 ± 43.20 pg/ml, while the average level in postmenopausal women was 44.18 ± 10.52 pg/ml. There were statistically significant differences between the levels of the two groups. However, it is worth noting that Reddy Kilim and Chandala collected samples for estradiol measurement on the 7th day of the menstrual cycle in premenopausal women, unlike the present study where samples were collected regardless of the day of the menstrual cycle. Several studies have examined the relationship between estradiol indicators and bone mineral density in postmenopausal women, either directly or indirectly [19, 20].
In terms of blood investigations, there were no statistically significant differences observed in serum calcium levels and all types of MCI. The majority of the body's calcium is kept in the skeletal system, making it a crucial vitamin for bone health. Calcium supplementation is considered an essential component of any therapy plan for osteoporosis. The findings of the current investigation were consistent with those of Okyay et al., who observed no statistically significant disparities in calcium levels between women with and without osteoporosis [41]. The findings of our investigation were consistent with the research conducted by Ramesh et al. In their study, they examined the relationship between average serum calcium. However, they did not see any statistically significant link between these variables [42]. Furthermore, our findings contradict the study conducted by Chandak et al, as there is limited evidence of a link between these indices and serum calcium [43].
In this study, there was a statistically significant difference between the two groups in terms of phosphate levels and vitamin D. These levels were higher in the post-menopausal group compared to the premenopausal group. This finding contradicts the previous study conducted by Balto et al, where no significant difference was found in the serum levels of LH, E2, calcium, phosphate, and 25(OH) D between the groups under investigation [39]. In another study conducted by Yadav et al, it was discovered that postmenopausal women had significantly lower levels of serum vitamin D and calcium (p<0.0001) compared to premenopausal women. On the other hand, the serum phosphorus level was significantly higher in postmenopausal women (p<0.0001) compared to premenopausal women [44].
The current investigation found no statistically significant difference in serum parathyroid hormone levels between the two groups. The PTH and vitamin D metabolites are crucial for maintaining calcium balance and regulating bone metabolism [44]. Our study found no significant difference in serum calcium levels between the two groups, indicating that there was also no significant difference in PTH levels.
Conclusion
The present study concludes that MCI, a panoramic radiographic parameter, can be utilised for both predicting and diagnosing osteoporosis in postmenopausal women. However, other radio-morphometric indices (PMI.MI) are not suitable for diagnosing osteoporosis. Instead, they can serve as supplementary tools to identify women with significant thinning or resorption of the inferior mandibular cortices, indicating the need for further investigation of osteoporosis. There is relation between the types of (MCI) and the levels of estradiol.
Conflict of Interests
The author has no conflict with any step of the articl e preparation
Consent for Publication
The author read and approved the final manuscript for publication.
Ethical Approval and Consent to Participation
All patients gave written and verbal consent before participation
References
- Celenk C, Celenk P. Relationship of mandibular and cervical vertebral bone density using computed tomography. Dentomaxillofac Radiol. 2008;37(1):47-51.
- Do Lee B, White SC. Age and trabecular features of alveolar bone associated with osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(1):92-8.
- Nackaerts O, Jacobs R, Horner K, Zhao F, Lindh C, Karayianni K, et al. Bone density measurements in intra-oral radiographs. Clin Oral Investig. 2007;11:225-9.
- Damilakis J, Vlasiadis K. Have panoramic indices the power to identify women with low BMD at the axial skeleton?. Physica Medica. 2011;27(1):39-43.
- Mays SA. Age-related cortical bone loss in women from a 3rd–4th century AD population from England. Am J Phys Anthropol. 2006;129(4):518-28.
- Cakur B, Dagistan S, Sahin A, Harorli A, Yilmaz AB. Reliability of mandibular cortical index and mandibular bone mineral density in the detection of osteoporotic women. Dentomaxillofac Radiol. 2009;38(5):255-61.
- Nakamoto T, Taguchi A, Ohtsuka M, Suei Y, Fujita M, Tsuda M, et al. A computer-aided diagnosis system to screen for osteoporosis using dental panoramic radiographs. Dentomaxillofac Radiol. 2008;37(5):274-81.
- Dutra V, Devlin H, Susin C, Yang J, Horner K, Fernandes AR. Mandibular morphological changes in low bone mass edentulous females: evaluation of panoramic radiographs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):663-8.
- Devlin H, Karayianni K, Mitsea A, Jacobs R, Lindh C, van der Stelt P, et al. Diagnosing osteoporosis by using dental panoramic radiographs: the OSTEODENT project. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(6):821-8.
- Taguchi A, Tsuda M, Ohtsuka M, Kodama I, Sanada M, Nakamoto T, et al. Use of dental panoramic radiographs in identifying younger postmenopausal women with osteoporosis. Osteoporos. 2006;17:387-94.
- Benson BW, Prihoda TJ, Glass BJ. Variations in adult cortical bone mass as measured by a panoramic mandibular index. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1991;71(3):349-56.
- Calciolari E, Donos N, Park JC, Petrie A, Mardas N. Panoramic measures for oral bone mass in detecting osteoporosis: a systematic review and meta-analysis. J Dent Res. 2015;94(3_suppl):17S-27S.
- Savic Pavicin I, Dumancic J, Jukic T, Badel T, Badanjak A. Digital orthopantomograms in osteoporosis detection: mandibular density and mandibular radiographic indices as skeletal BMD predictors. Dentomaxillofac Radiol. 2014;43(7):20130366.
- Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Case-control study on self-reported osteoporotic fractures and mandibular cortical bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(4):518-24.
- Edlefsen KL, Jackson RD, Prentice RL, Janssen I, Rajkovic A, O'Sullivan MJ, et al. The effects of postmenopausal hormone therapy on serum estrogen, progesterone, and sex hormone-binding globulin levels in healthy postmenopausal women. Menopause. 2010;17(3):622-9.
- Cervellati C, Bonaccorsi G, Cremonini E, Bergamini CM, Patella A, Castaldini C, et al. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin Chem Lab Med. 2013;51(2):333-8.
- Morcov C, Vulpoi C, Branisteanu D. Relationship between bone mineral density, weight, and estrogen levels in pre and postmenopausal women. J Surg. 2012;116(4):946-50.
- Svejme O, Ahlborg HG, Karlsson MK. Changes in forearm bone mass and bone size after menopause. A mean 24-year prospective study. J Musculoskelet Neuronal Interact. 2012;12(4):192-8.
- Kilim SR, Chandala SR. A comparative study of lipid profile and oestradiol in pre-and post-menopausal women. J Clin Diagn Res. 2013;7(8):1596.
- Napoli N, Varadharajan A, Rini GB, Del Fiacco R, Yarramaneni J, Mumm S, et al. Effects of polymorphisms of the sex hormone-binding globulin (SHBG) gene on free estradiol and bone mineral density. Bone. 2009;45(6):1169-74.
- Lanham-New SA. Role of calcium and vitamin D in the prevention (and treatment) of osteoporotic fracture. Surgery. 2009;27(2):47-54.
- Marino M, Masella R, Bulzomi P, Campesi I, Malorni W, Franconi F. Nutrition and human health from a sex–gender perspective. Mol Asp Med. 2011;32(1):1-70..
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin. 2008;87(4):1080S-6S.
- Vitamin D. deficiency. Holick MF. N Engl J Med. 2007;357:266-81.
- Girgis CM, Clifton-Bligh RJ, Hamrick MW, Holick MF, Gunton JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013;34(1):33-83.
- Lundgren E, Rastad J, Thurfjell E, Åkerström G, Ljunghall S. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121(3):287-94.
- Wishart J, Horowitz M, Need A, Nordin BE. Relationship between forearm and vertebral mineral density in postmenopausal women with primary hyperparathyroidism. Arch Intern Med. 1990;150(6):1329-31.
- Sneddon WB, Magyar CE, Willick GE, Syme CA, Galbiati F, Bisello A, et al. Ligand-selective dissociation of activation and internalization of the parathyroid hormone (PTH) receptor: conditional efficacy of PTH peptide fragments. Endocrinol. 2004;145(6):2815-23.
- van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ. Coordinated control of renal Ca2+ transport proteins by parathyroid hormone. Kidney Int. 2005;68(4):1708-21.
- Klemetti E, Kolmakov S, Kröger H. Pantomography in assessment of the osteoporosis risk group. Eur J Oral Sci. 1994;102(1):68-72.
- Asha ML, Bajoria AA, Babshet M, Patil P, Naveen S. Bone mineral density measurement of the jaws–a review. J Investigative Dent Sci. 2014;1(1):0000004.
- Gu¨ngo¨r KA, Akarslan ZZ, Akdevelioglu M, Erten H, Semiz M. The precision of the panoramic mandibular index. Dentomaxillofac Radiol. 2006;35(6):442-6.
- Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, et al. Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. Am J Roentgenol. 2004;183(6):1755-60.
- White SC. Oral radiographic predictors of osteoporosis. Dentomaxillofac Radiol. 2002;31(2):84-92.
- Mudda JA, Bajaj M, Patil VA. A Radiographic comparison of mandibular bone quality in pre-and post-menopausal women in Indian population. J Indian Soc Periodontol. 2010;14(2):121-5.
- Knezovic Zlataric D, Celebic A, Lazic B, Baucic I, Komar D, Stipetic-Ovcaricek J, et al. Influence of age and gender on radiomorphometric indices of the mandible in removable denture wearers. Coll Antropol. 2002;26(1):259-66.
- Horner K, Devlin H, Harvey L. Detecting patients with low skeletal bone mass. J Dent. 2002;30(4):171-5.
- Dutra V, Susin C, da Costa NP, Veeck EB, Bahlis A, Fernandes AD. Measuring cortical thickness on panoramic radiographs: a validation study of the Mental Index. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Nov 1;104(5):686-91.
- Balto KA, Gomaa MM, Feteih RM, AlAmoudi NM, Elsamanoudy AZ, Hassanien MA, et al. Dental panoramic radiographic indices as a predictor of osteoporosis in postmenopausal Saudi women. J Bone Metab. 2018;25(3):165.
- GauR B, Chaudhary A, Wanjari PV, Sunil MK, Basavaraj P. Evaluation of panoramic radiographs as a screening tool of osteoporosis in post menopausal women: a cross sectional study. J Clin Diagn Res. 2013;7(9):2051.
- Okyay E, Ertugrul C, Acar B, Sisman AR, Onvural B, Ozaksoy D. Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas. 2013;76(4):320-5.
- Chandak LG, Lohe VK, Bhowate RR, Gandhi KP, Vyas NV. Correlation of mandibular radiomorphometric indices with serum calcium and serum estradiol in pre-and post-menopausal women. Contemp Clin Dent. 2017;8(1):53-8.
- Yadav KP, Batra J, Singh UN, Yadav R. Serum vitamin d, serum calcium & serum phosphorus in post-menopausal women in Farrukhabad District, Uttar Pradesh, India. AIMDR. 2020;6(1):1-4.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol. 2008;156(1):1-8.
1Lecturer, Department of Oral Diagnosis, College of Dentistry, Hawler Medical University, Erbil, Kurdistan-Region, Iraq
2Assistant Professor, Department of Oral Diagnosis, College of Dentistry, Hawler Medical University, Erbil, Kurdistan-Region, Iraq
3Assistant Lecturer, Department of Oral Diagnosis, College of Dentistry, Hawler Medical University, Erbil, Kurdistan-Region, Iraq
4Professor and Consultant in Oral and Maxillofacial Medicine and Surgery, Kurdistan Higher Council of Medical Specialties, Erbil-Iraq
Send correspondence to:
Asma Qasim Rahman
Lecturer, Department of Oral Diagnosis, College of Dentistry, Hawler Medical University, Erbil, Kurdistan-Region, Iraq, E-mail: asmaa.qasim@hmu.edu.krd
Paper submitted on Jul 26, 2024; and Accepted on Aug 12, 2024
Citation: Asma Qasim Rahman. Evaluation of Osteoporosis in Postmenopausal Women by Dental Panoramic Radiographic Indices as a Predictor of Osteoporosis and Relation to Some Serum Biochemical Markers: A Case Control Study. Int Tinnitus J. 2024;28(1):177-186


