The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 27, Issue 1 / June 2023
Research Article Pages:10-15
10.5935/0946-5448.20230003
Imbalance of Follicular Helper and Follicular Regulatory T Cells in Chronic Rhinosinusitis with Nasal Polyps
Authors: Asmaa M. Zahran, Omnia El-Badawy, Eman Hosny M. Ibrahim, Khaled Saad, Abobakr Abdelmoghny, Amira Elhoufey, Hamad Ghaleb Dailah, Mohamed M. Osman
PDF
Abstract
Objective: Data regarding the imbalance in follicular helper T (Tfh) and follicular regulatory T (Tfr) cell responses in patients having chronic rhinosinusitis with nasal polyps (CRSwNP) is so far limited. Thus, we aimed to assess the changes in circulating Tfh and Tfr in CRSwNP patients.
Methods: This case-control study included 21 patients having CRSwNP and 20 age and sex-matched healthy blood donors as a control group. Lund-Mackay staging system was used for radiologic scoring of chronic rhinosinusitis. Two milliliters of peripheral blood samples were collected from all participants into EDTA-containing vacutainer tubes to assess the levels of Tfh and Tfr cells using flow cytometry.
Results: Patients having CRSwNP did not show significant differences in the percentages of CD4+ T cells and total CD4+CXCR5+ T cells from healthy controls. Meanwhile, levels of both activated circulating Tfh and Tfr showed a marked rise in patients than controls. In addition, a positive correlation was observed between the levels of both activated Tfh and Tfr cells.
Conclusion: An imbalance in circulating Tfh/Tfr levels was detected in patients having CRSwNP. A significant rise in the levels of Tfh and Tfr was detected in patients proposing a possible role of this imbalance in disease pathogenesis.
Keywords: Follicular helper T cells, Follicular regulatory T cells, Chronic rhinosinusitis, Nasal polyps.
Introduction
Chronic Rhinosinusitis (CRS) is a complex heterogeneous disease distinguished by sinonasal chronic inflammation. Endoscopic examination and imaging procedures of the nasal cavity classify CRS phenotypically into Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) and Chronic Rhinosinusitis without Nasal Polyps (CRSsNP). Nasal polyps appear as visible sinonasal inflammatory masses that gradually obstruct nasal airflow and sinus drainage [1].
The diversity of T cell subtypes has been described in chronic sinusitis and nasal polyposis, and their biological function has been identified. CD4+ T cells may differentiate into one of several lineages, including T helper cells (Th) 1, Th2, Th17, Th9, and follicular helper T (Tfh) cells [2, 3]. The equilibrium between these T-cell subsets is essential for the mucosal physiological immune response, which is dysregulated by persistent inflammation [4].
A distinctive feature of CRSwNP is the prevalence of eosinophils and Th2 cytokines in the sinus mucosa [5]. The factors implicated in inducing this mucosal eosinophilia remain largely unknown. Nevertheless, elevated IgE production in polyp tissues mediates mucosal mast cell activation and subsequently causes eosinophilic inflammation [6]. Even though Th2 cells have long been regarded as the primary cell type inducing B cell classswitching to IgE, more recent studies showed that this process mainly depends on Tfh cells [7].
Tfh cells are a specialized subpopulation of CD4+ T cells that help B cells, promoting Germinal Central (GC) responses in B cell follicles of secondary lymphoid organs [8, 9]. Besides, Tfh cells have also been recognized in peripheral blood. They share phenotypic and functional similarities with their bona fide Tfh cells in secondary lymphoid organs and predominantly have a memory phenotype [10]. Three subsets of circulating Tfh have been identified; Tfh1, Tfh2, and Tfh17 cells, which secrete cytokines produced by the conventional helper-T cell subsets [11]. Between Tfh subsets, the Tfh2 cells are considered the primary producer of interleukin (IL)-4, promoting the B cell class-switching to IgE. Increased levels of circulating or tissue Tfh2 cells have been described in some inflammatory airway disorders, such as allergic rhinitis, nasal polyposis, and asthma [12, 13].
Follicular regulatory T cells (Tfr) are a unique population of regulatory T cells (Tregs) that suppress GC responses by inhibiting Tfh cell activation [14, 15]. Phenotypically, Tfr cells co-express the Tfh and Treg cell’s characteristic markers [16]. Data regarding the imbalance in Tfh/Tfr cell responses in patients with CRSwNP is limited. Thus, we aimed to assess the changes in the levels of circulating Tfh and Tfr in CRSwNP patients.
Methods
The current case-control study included 21 patients having CRSwNP enrolled from the Department of Otorhinolaryngology & Head and Neck Surgery, Assiut University. Twenty age and sex-matched healthy blood donors were also recruited as a control group. The Ethics Committee of the Faculty of Medicine, Assiut University, appraised and accepted the study protocol (IRB. No.17300110) following the Helsinki Declaration. Written informed consent was collected from all patients before participation.
For radiologic evaluation of chronic rhinosinusitis, a noncontrast computed tomography was performed using standard bone and soft-tissue window settings, coronal, axial, and sagittal cross-sections of 3 mm thickness. Lund-Mackay staging system was used for chronic rhinosinusitis scoring [17]. Each sinus was assigned a value of 0 to 2; 0 for totally patent, 1 for partially opacified, or 2 for wholly opacified. The osteomeatal complex (OMC) was given a score of either 0 or 2 (not occluded or occluded, respectively). The maximum score for each side is hence 12, with a total score up to 24 (all sinuses are completely opacified). Lund Mackay scores of 4 and above broadly support the clinical diagnosis of CRS [18].
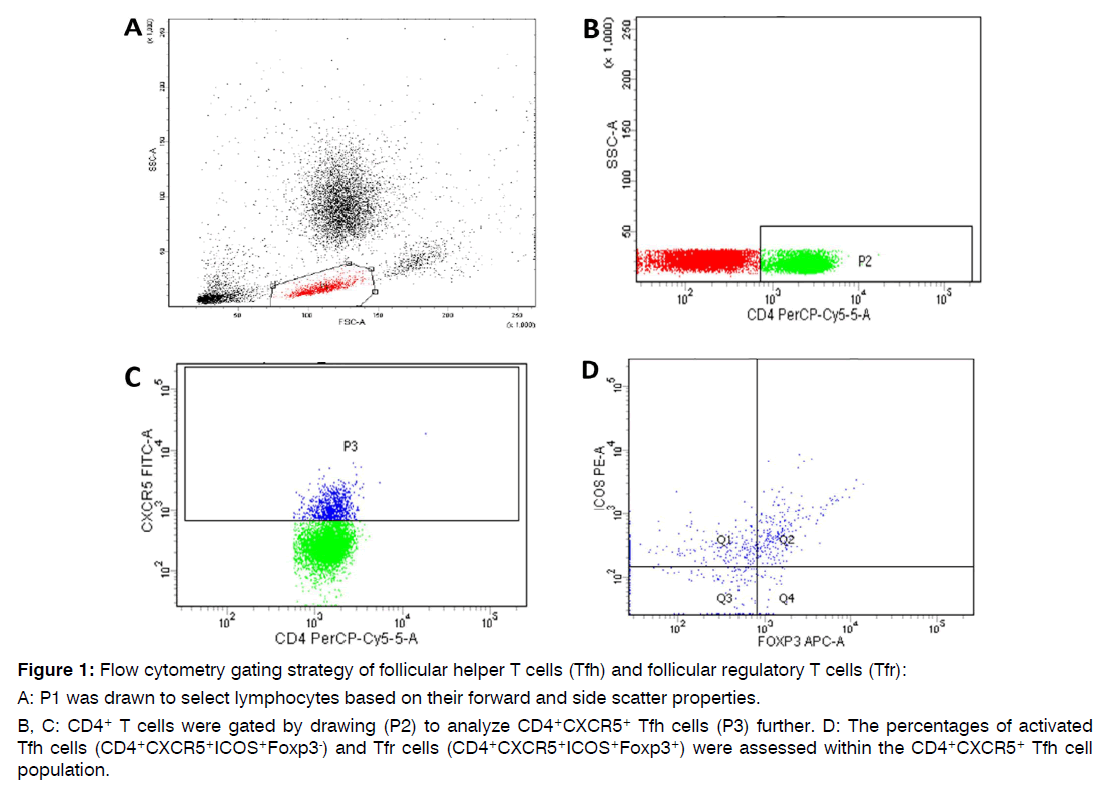
Flow Cytometric Assessment of Follicular Helper and Follicular Regulatory T Cells: Preoperatively, two milliliters of peripheral blood samples were obtained from all participants into EDTA containing vacutainer tubes to assess the levels of Tfh and Tfr cells using flow cytometry. First, 100 μl of whole blood samples were incubated for 20 minutes with 10 μl of Fluoroisothiocyanate (FITC)-conjugated anti-CXCR5 (R&D systems, USA), Phycoerythrin (PE) conjugated anti-ICOS (R&D systems, USA), and Peridinium-Chlorophyll-Protein (PerCP)-Cy5.5- conjugated anti-CD4 (R&D systems, USA), monoclonal antibodies. Next, red blood cells were lysed, then samples were washed with Phosphate-Buffered Saline (PBS). Fixation solution was added with 10-minute incubation. After PBS rewash, 10 μl of Allophycocyanin (APC)-conjugated anti-Foxp3 (eBioscience, USA) were added together with the permeabilizing solution and incubated for 20 minutes. Anti-human IgG isotypematched negative control was used for each sample to evaluate the non-specific staining. Nearly 50,000 events were collected and analyzed by the FACSCanto flow cytometer using FACSDiva software (Becton Dickinson Biosciences, USA), South Egypt Cancer Institute, Assiut University. Figure 1 illustrates the gating strategy used for the study. The total Tfh cells were defined as CD4+CXCR5+ T lymphocytes, activated Tfh cells as CD4+CXCR5+ICOS+Foxp3-, and Tfr cells as CD4+CXCR5+ICOS+Foxp3+.
Figure 1: Flow cytometry gating strategy of follicular helper T cells (Tfh) and follicular regulatory T cells (Tfr):
A: P1 was drawn to select lymphocytes based on their forward and side scatter properties.
B, C: CD4+ T cells were gated by drawing (P2) to analyze CD4+CXCR5+ Tfh cells (P3) further. D: The percentages of activated Tfh cells (CD4+CXCR5+ICOS+Foxp3-) and Tfr cells (CD4+CXCR5+ICOS+Foxp3+) were assessed within the CD4+CXCR5+ Tfh cell population.
Statistical Analysis: IBM Statistical Package for the Social Sciences (IBM SPSS statistics, version 20) was used for the statistical analysis. Categorical data were presented as numbers and percentages, while quantitative data were expressed as the mean and standard error. The Mann-Whitney U test was applied to compare groups, and Spearman rank-order correlation was employed to evaluate the association between different variables. P-value is significant if <0.05.
Results
Patients’ Laboratory and Radiologic Features: The mean age of the enrolled CRSwNP patients was 32.6±4. The average total leukocyte count was 7±0.5X106/L, Neutrophil count was 3.9±0.5X106/L (53.3±3%), and eosinophil count was 4±0.8/L (0.3±0.05%). Total radiologic scores varied from 8 to 24, and about half of the patients had a score greater than 18. Laboratory and radiologic features of patients are demonstrated in the Table 1.
| Variables | Patients (n=21) | Control (n=20) |
|---|---|---|
| Age (years) | 32.6 ± 4 | 36 ± 2 |
| Sex Males Females |
14 (66.7%) 7 (33.3%) |
13 (65%) 7 (35%) |
| Hematological findings TLC (X106/L) Neutrophil percent/ count (X106/L) Eosinophil percent/count (X106/L) |
7 ± 0.5 53.3 ± 3/3.9 ± 0.5 4 ± 0.8/0.3 ± 0.05 |
7.5 ± 0.4 50.6 ± 2/3.4 ± 0.2 2.7 ± 0.4/0.2 ± 0.04 |
| Total radiologic score 8 13 14 15 16 18 19 21 22 24 |
2 (9.5%) 1 (4.8%) 2 (9.5%) 1 (4.8%) 1 (4.8%) 4 (19%) 1 (4.8%) 1 (4.8%) 2 (9.5%) 6 (28.6%) |
NA |
Table 1: Patients' Laboratory and radiologic features.
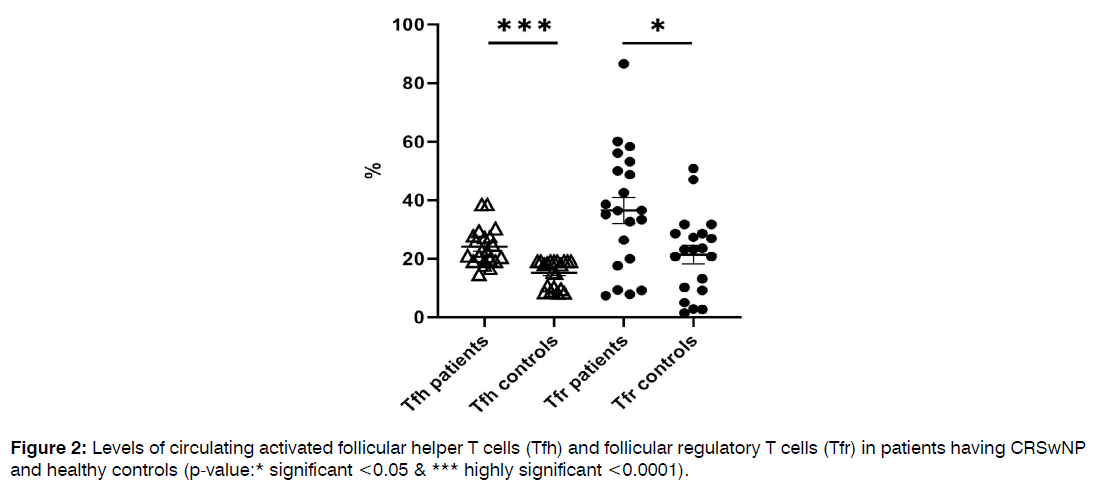
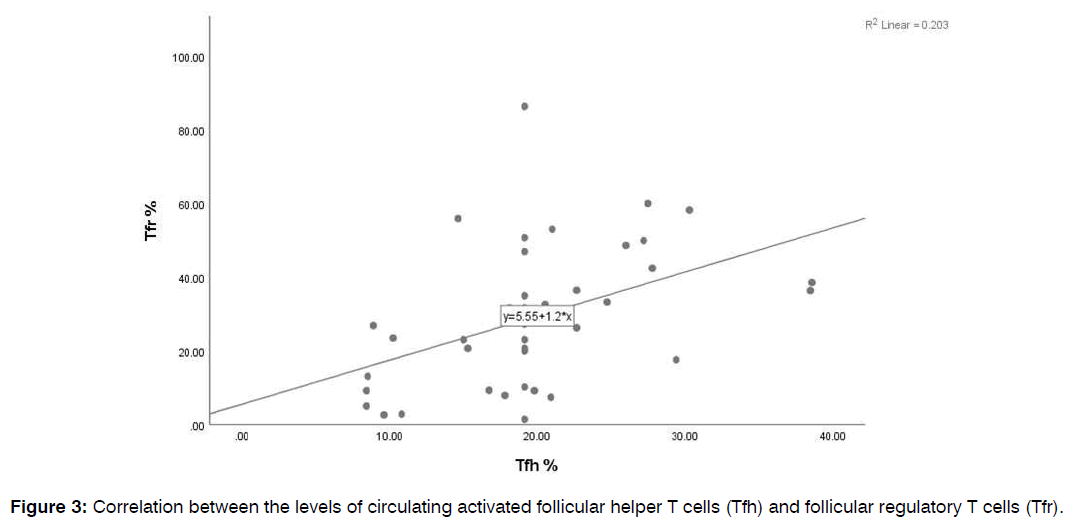
Comparison of the Frequencies of Circulating Follicular Helper and Follicular Regulatory T Cells in Patients and Healthy Controls: Patients having CRSwNP didn’t show significant differences in the percentages of CD4+ T cells and total CD4+CXCR5+ Tfh cells from healthy controls (34.6±2 vs. 38.7±1, p=0.2 and 8.9±1 vs. 9.3±1, p=0.5, respectively). Meanwhile, levels of both activated Tfh and Tfr showed a marked rise in patients than controls (24±1 vs. 15±1, p<0.0001 and 36.4±4 vs. 21.4±3, p=0.01), as illustrated in Figure 2. In addition, a positive correlation was observed between the levels of both activated Tfh and Tfr cells (r=0.4, p<0.0001), Figure 3. Tfh and Tfr cells didn’t show any significant correlations with either eosinophil count or CT score.
Figure 2: Levels of circulating activated follicular helper T cells (Tfh) and follicular regulatory T cells (Tfr) in patients having CRSwNP and healthy controls (p-value:* significant <0.05 & *** highly significant <0.0001).
Figure 3: Correlation between the levels of circulating activated follicular helper T cells (Tfh) and follicular regulatory T cells (Tfr).
Discussion
Data regarding the imbalance in Tfh and Tfr cell responses in patients having CRSwNP is so far limited. Thus, we aimed to assess the changes in circulating Tfh and Tfr in CRSwNP patients. The CRSwNP group didn’t show significant differences in the percentages of CD4+ T cells and total CD4+CXCR5+ Tfh cells from healthy controls. Meanwhile, levels of both activated Tfh and Tfr showed a marked rise in patients than controls. In addition, a positive correlation was observed between the levels of both activated Tfh and Tfr cells.
CRSwNP has been considered a Th2-mediated inflammation, characterized by high IL-4, IL-5, and eosinophilic inflammation. In comparison, CRSsNP is predominantly Th1 inflammation, with increased expression of interferon-gamma (IFN-γ) and Transforming Growth Factor-beta (TGF-β). A previous study reported that Th2 cells within the sinonasal mucosa of CRS patients were only detected in patients with nasal polyposis and were higher in patients having asthma and/ or Staphylococcus aureus enterotoxin specific IgE [19].
The central role of type-2 cytokines in the pathogenesis of CRSwNP is emphasized by the excellent clinical efficacy of dupilumab, a fully humanized IgG4 monoclonal antibody against the IL-4 receptor α (IL-4Rα) subunit [20]. Yet, the relevant concepts have gradually become more complex, since authors described the probable involvement of other T cell subsets, rather than Th2, as Th17 and Th22 in the pathogenesis of CRSwNP [21].
Emerging evidence suggests that IgE class switch recombination largely relies on Tfh cells [7, 22]. In addition, patients with allergic diseases have shown an upregulation of Tfh cell activity, including a Tfh phenotype with skewed differentiation toward Tfh2 cells and IL-13-producing Tfh13 cells [23].
An earlier study [24], showed that a higher frequency of B-cell lymphoma-6 (Bcl-6)+CD4+ Tfh cells in polyp tissues was accompanied by B cell accumulation, activation and IgA and IgE production in polyp tissue. They also found that levels Bcl-6+CD4+ cells and immunoglobulins were significantly higher in CRSwNP patients with GC-like structure than those without GC-like structure. Additionally, the frequencies of Bcl-6+CD4+ cells correlated with the tissue eosinophilia, asthma comorbidity and recurrence after surgery, suggesting that Tfh cells may present a potential biomarker of disease severity.
In another study [25], a significant increase was observed in the levels of total Tfh cells and Tfh cell subsets secreting IL-21, IFN-γ, and IL-17 in both eosinophilic and noneosinophilic nasal polyp tissues than in normal nasal tissues. However, Tfh cells secreting IL-4 have only increased in the eosinophilic nasal polyp tissues and strongly correlated with the local IgE levels. Authors deduced that IL-4 and IL-21 were implicated in polyp Tfh cell-induced IgE production. Moreover, Bcl-6+ Tfh cells secreting IL-4 were found in ectopic lymphoid tissue in eosinophilic nasal polyps. Tfh cells are also directly correlated with the GC B cells and plasma cells in the nasal tissues. On the contrary, they didn’t detect meaningful differences in the frequencies of total Tfh cells, most of the Tfh cell subsets, and B cells in peripheral blood among the studied groups. On the other hand, another study showed no differences in CD4+ T cell, activated CD4+ T cell, CD8+T cell, Tfh cells, Tregs, B cells, and IgA+ B cells between nasal polyp tissue and sinus mucosa [26].
IL-21 mRNA expression was upregulated in the CRSwNP group compared to the control group, and Bcl-6 and B-lymphocyte-induced maturation protein-1 were elevated in CRSwNP versus CRSsNP. Furthermore, IL-21 mRNA expression and Tfh cells secreting IL-21 increased significantly in nasal polyp tissue and increased more after stimulation with S. aureus enterotoxin B. They concluded that Tfh cells and IL-21 are central in the pathophysiology of CRSwNP [27].
Lately, Bergantini and coauthors reported higher levels of total Tfh and Tfh2 and lower Tfr in peripheral blood of patients with nasal polyps than the controls. They also noticed a significant reduction of Tfh and Tfh2 frequencies after six months of Omalizumab therapy, a humanized IgG1k monoclonal antibody against IgE [28].
The Tfr cells, in B cell follicles, restrain Tfh-mediated help in B-cell activation, IgM, IgA, IgG, and IgE production, and GC responses that give rise to long-lived plasma cell and affinity-matured memory B cell differentiation [22]. The increased levels of both circulating Tfh and Tfr observed in our patients, in addition to the positive correlation between the two cells, might be a counter-regulatory mechanism in a trial to control the increased Tfh numbers in those patients. This explanation was in line with that in a previous SLE study but needed further investigations.
Conclusion
An imbalance in circulating Tfh/Tfr levels was detected in patients having CRSwNP. Levels of both Tfh and Tfr were elevated in patients proposing a possible role of this imbalance in disease pathogenesis.
Conflict of Interest
The authors declare having no conflict of interests.
Role of Funding
This work was not supported by grants from any funding agency.
Data Availability Statements
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request after taking permission from our ethical committee.
References
- Kariyawasam HH, Rotiroti G. Allergic rhinitis, chronic rhinosinusitis and asthma: unravelling a complex relationship. Curr Opin Otolaryngol Head Neck Surg. 2013;21(1):79-86.
- Zahran AM, Abdallah AM, Saad K, Osman NS, Youssef MA, Abdel-Raheem YF, et al. Peripheral blood B and T cell profiles in children with active juvenile idiopathic arthritis. Arch Immunol Ther Exp. 2019;67(6):427-32.
- Elsayh KI, Saad K, Hetta HF, Youssef MA, Embaby MM, Mohamed IL, et al. Impact of hydroxyurea on lymphocyte subsets in children with sickle cell anemia. Pediatr Res. 2021:1-6.
- Fokkens W, Lund V, Bachert C, Clement P, Helllings P, Holmstrom M, et al. European position paper on rhinosinusitis and nasal polyps. Rhinol. 2005;23(23):1-87.
- Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6):155-212.
- Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non?eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014;44(5):690-700.
- Zahran AM, Abdel-Rahim MH, Nasif KA, Hussein S, Hafez R, Ahmad AB, et al. Association of follicular helper T and follicular regulatory T cells with severity and hyperglycemia in hospitalized COVID-19 patients. Virulence. 2022;13(1):569-77.
- Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol. 2012;9(5):375-9.
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324-35.
- Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436-42.
- Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5+ CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108-21.
- Yao Y, Wang ZC, Yu D, Liu Z. Role of allergen-specific T-follicular helper cells in immunotherapy. Curr Opin Allergy Clin Immunol. 2018;18(6):495-501.
- Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015;158(2):204-11.
- Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, et al. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17(12):1436-46.
- Sage PT, Sharpe AH. T follicular regulatory cells. Immunol Rev. 2016;271(1):246-59.
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983-8.
- Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993;31:183-4.
- Bhattacharyya N, Fried MP. The accuracy of computed tomography in the diagnosis of chronic rhinosinusitis. The Laryngoscope. 2003;113(1):125-9.
- Derycke L, Eyerich S, Van Crombruggen K, Pérez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PloS one. 2014;9(6):e97581.
- Hoy SM. Dupilumab: A review in chronic rhinosinusitis with nasal polyps. Drugs. 2020;80(7):711-7.
- Carsuzaa F, Béquignon É, Dufour X, de Bonnecaze G, Lecron JC, Favot L. Cytokine signature and involvement in chronic rhinosinusitis with nasal polyps. Int J Mol Sci. 2022;23(1):417.
- Yao Y, Chen CL, Yu D, Liu Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy. 2021;76(2):456-70.
- Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365(6456):eaaw6433.
- Wan Y, Bai Y, Sun S, Qiu X, Zheng Y, Wang H, et al. Increased expression of transcription factor Bcl-6 in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol. 2016;273(2):391-9.
- Zhang YN, Song J, Wang H, Wang H, Zeng M, Zhai GT, et al. Nasal IL-4+ CXCR5+ CD4+ T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps. J Allergy Clin Immunol. 2016;137(2):462-73.
- Calus L, Derycke L, Dullaers M, Van Zele T, De Ruyck N, Pérez-Novo C, et al. IL-21 is increased in nasal polyposis and after stimulation with Staphylococcus aureus enterotoxin B. Int Arch Allergy Immunol. 2017;174(3-4):161-9.
- Bergantini L, d’Alessandro M, Cameli P, Pianigiani T, Fanetti M, Sestini P, et al. Follicular T helper and Breg cell balance in severe allergic asthma before and after omalizumab therapy. Mol Diagn Ther. 2021;25(5):593-605.
- Liu C, Wang D, Song Y, Lu S, Zhao J, Wang H. Increased circulating CD4+ CXCR5+ FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol. 2018;56:261-8.
1Department of Clinical Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
2Department of Medical Microbiology & Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt
3Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt
4Department of ENT, Faculty of Medicine, Al-Azhar University, Assiut, Egypt
5Department of Community Health Nursing, Faculty of Nursing, Assiut University, Assiut, Egypt
6Research and Scientific Studies Unit, College of Nursing, Jazan University, Jazan, Saudi Arabia
7Department of Otorhinolaryngology & Head and Neck Surgery, Faculty of Medicine, Assiut University, Assiut, Egypt
Send correspondence to: Khaled Saad Professor, Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt, E-mail: Khaled.ali@med.au.edu.eg Tel no: 1097075321
Paper submitted on November 21, 2022; and Accepted on January 18, 2023
Citation: Zahran AM, El-Badawy O, Ibrahim EHM, Saad K, Abdelmoghny A, Elhoufey A, et al. Imbalance of Follicular Helper and Follicular Regulatory T Cells in Chronic Rhinosinusitis with Nasal Polyps. Int Tinnitus J. 2023;27(1):10-15.