The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 27, Issue 1 / June 2023
Research Article Pages:61-67
10.5935/0946-5448.20230011
Is Topical Nasal steroid Useful for Treatment of Otitis Media with Effusion in Children?
Authors: Ahmed Muhei Rasheed
PDF
Abstract
Background: Otitis media with effusion is a common and important pediatric clinical problem; it is the leading cause of hearing impairment in children. Medical treatment remains controversial.
Aim: To evaluate the usefulness of using topical nasal steroids in the treatment of otitis media with effusion.
Patients and Methods: Between November 2019 and October 2022, a prospective controlled clinical study was carried out in the department of otolaryngology at Al-Jerrahat Teaching Hospital in Medical City, Baghdad, Iraq. This study comprised 40 patients with bilateral otitis media with effusion (23 males, 17 females). Two groups were created for the patients. Patients in group A (20 patients) were treated with mometasone furoate nasal spray; 1 puff (50 µg) in each nostril daily for 2 weeks, while the 20 patients in group B were treated with saline nasal spray; 1 puff in each nostril daily for 2 weeks. At the end of the first and second weeks of treatment, otoscopic examination was used to monitor the patients. At the end of the second post-treatment week, pure tone audiometry and tympanometry were performed again. Normal otoscopic results, a type A tympanogram, and enhanced pure tone hearing threshold average to be ≤20 dB HL within 0, 5, 1, and 4 KHz were used to characterize resolution of OME. The association between two means was determined using an independent sample t-test, while the association between categorical variables was determined using an X2-test.
Results: At the end of 2nd post-treatment week, there was no significant difference regarding improvement of otitis media with effusion regarding otoscopic, audiometric, and tympanometric results in both groups (P-value >0.05). Conclusion: Topical nasal steroid is unuseful for the treatment of otitis media with effusion in the short-term.
Keywords: Otitis media with effusion, Topical nasal steroid, Audiometry, Tympanometry
Introduction
Otitis Media with Effusion (OME) is defined as a condition characterized by the presence of middle ear fluid with the absence of signs of acute infection [1]. Passive smoking, atopy, bottle feeding, day- care nursery are recognizable risk factors for OME [2]. Enlarged adenoid and upper respiratory tract infection can lead to OME [3]. The incidence of OME is more common in children than adults due to the more horizontal position of the Eustachian tube, as well as the position of the head in children [4]. Children with developmental anomalies including decreased palatal muscle tone, or bone developmental variations are at higher risk of OME development, e.g. cleft palate, down syndrome [5]. Furthermore, in children with Down syndrome there is impaired mucociliary function that increases the risk for OME development [6].
By the age of 10 years, about 80% of children have had one or more episodes of OME [7]. Hearing impairment is the most common complaint in patients with OME. Other complaints may include communication difficulties, lack of attention, impaired speech development, and sensation of aural fullness. Signs of OME include tympanic membrane opacification, loss of light reflex, and retracted tympanic membrane with impaired mobility [8].
Patients with OME can be evaluated by using ageappropriate audiometry and tympanometry. Type B tympanogram will support the diagnosis of OME [9]. Generally, OME resolves spontaneously with watchful waiting. In persistent OME, myringotomy with the insertion of a tympanostomy tube is considered an effective treatment [10]. However, there is no general agreement about the medical treatment of OME, e.g. steroids, and antibiotics. Therefore, this study was conducted to evaluate the usefulness of using topical nasal steroids (mometasone furoate nasal spray) in the treatment of OME.
Patiends and Methods
The Helsinki Declaration was followed to gain ethical concerns. After describing the purpose of the study to the parents, formal informed consent was acquired. The health ethics committee of the college of medicine at the University of Baghdad granted the ethics committee’s permission.
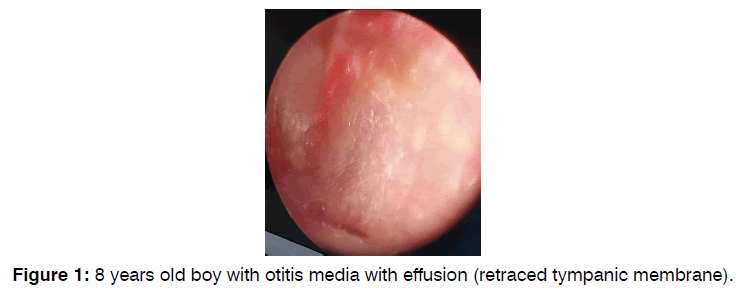
40 patients with bilateral OME (Figure 1) attended the department of otolaryngology at Al-jerahat Teaching Hospital in Medical City, Baghdad, Iraq; during the period from November 2019 to October 2022 who met the inclusion criteria were included in the current prospective controlled clinical study.
The patients were subjected to routine otolaryngological examination including otoscopic examination, fiber optic nasal endoscopy, pure tone audiometry, tympanometry, and plain x-ray of postnasal space (Figure 2).
The inclusion criteria were:
1. Age 6-10 years.
2. Bilateral OME for at least 3 months.
Average pure tone hearing threshold of more than 20 dB HL within frequencies 0.5, 1, 2, and 4 KHz.
Type B tympanogram.
The exclusion criteria were:
1. Symptomatic adenoid enlargement.
2. A history of steroid use in the prior three months.
3. Adenoidectomy and/or myringotomy history, with or without insertion of a tympanostomy tube.
4. A current ear infection or upper respiratory illness.
5. Cleft palate, down syndrome, Kartagener syndrome, or Young syndrome.
6. Immunocompromised diseases e.g., diabetes mellitus.
The patients who met the entry criteria were given code numbers. Group A patients, including 20 children, were given odd numbers, while the 20 patients in group B were given double numbers. Patients in group A were treated with mometasone furoate nasal spray; one puff in each nostril (100 μg/day), while patients in group B received saline nasal spray; one puff in each nostril/day. The duration of treatment was 2 weeks in both groups.
At the end of the first and second weeks of treatment, otoscopic examination was used to monitor the patients.
At the end of the second post-treatment week, pure tone audiometry and tympanometry were performed again. Normal otoscopic results, a type A tympanogram, and enhanced pure tone hearing threshold average to be ≤ 20 dB HL within 0, 5, 1, and 4 KHz were used to characterize resolution of OME. The tympanometry results were classified into types A, B, and C according to Modified Jerger Classification.
Statistical Analysis
1. The statistical software for social sciences version 23 was used to analyze the data.
2. The association between two means was defined using independent samples t-test.
3. The association between the category variables was determined using the X2-test.
4. A 5% error margin was accepted and a 95% confidence level was assigned.
5. P-value ≤ 0.05 was considered significant.
Results
The total number of patients enrolled in this study was 40 patients (80 ears) with ages ranging from 6 to 10 years (mean=7.45 years ± SD 1.32). 23 patients (57.5%) were male and 17 patients (42.5%) were female, male: female ratio=1.4:1. There was no significant difference between both groups regarding age and gender (P-value >0.05) as shown in Tables 1 and 2 respectively.
| Age (years) | Group A | Group B | Total | Mean difference | Std. Error difference | t-test | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | ||||||||||
| 6 | 6 | 15 | 7 | 17.5 | 13 | 32.5 | 0.8 | 0.524 | 1.524 | 1.36 | |||||
| 7 | 5 | 12.5 | 4 | 10 | 9 | 22.5 | |||||||||
| 8 | 3 | 7.5 | 5 | 12.5 | 8 | 20 | |||||||||
| 9 | 4 | 10 | 3 | 7.5 | 7 | 17.5 | |||||||||
| 10 | 2 | 5 | 1 | 2.5 | 3 | 7.5 | |||||||||
| Total | 20 | 50 | 20 | 50 | 40 | 100 | |||||||||
Table 1: Age distribution.
| Sex | Group A | Group B | Total | X2 | P-value | |||
|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |||
| Male | 11 | 27.5 | 12 | 30 | 23 | 57.5 | 0.1 | >0.05 |
| Female | 9 | 22.5 | 8 | 20 | 17 | 42.5 | ||
| Total | 20 | 50 | 20 | 50 | 40 | 100 | ||
Table 2:. Gender distribution.
There was no significant difference between group A and group B regarding pre-treatment otoscopic findings and pure tone hearing threshold average within 0.5, 1, 2, and 4 KHz as shown in Tables 3 and 4 respectively. Tympanograms were flat (type B) in all patients included in the study.
| Otoscopic Findings | Group A Number of Ears (40) & % | Group B Number of Ears (40) & % | X2 | P-value |
|---|---|---|---|---|
| Retracted tympanic membrane | 40 (100%) | 40 (100%) | 0.66 | >0.05 |
| Opaque tympanic membrane | 35 (87.5%) | 37 (92.5%) | ||
| Air fluid level | 4 (10%) | 2 (5%) | ||
| Air bubbles | 2 (5%) | 2 (5%) |
Table 3: Pre-treatment otoscopic examination.
| Pure tone hearing threshold means ± SD | Independent samples t-test | P-value | |
|---|---|---|---|
| Group A | Group B | ||
| 26.85 ± 0.489 | 26.6 ± 0.502 | 1.594 | 0.119 |
Table 4: Means of pre-treatment pure tone hearing threshold average within 0.5, 1, 2, and 4 KHz.
The pre-treatment pure tone audiogram and tympanogram of two patients included in the study are shown in Figures 3 and 4 respectively. Statistical analysis had shown that there was no significant difference between group A and group B patients at the end of 2nd post-treatment week regarding otoscopic, tympanometric, and audiometric results as shown in Tables 5, 6, and 7 respectively.
| Otoscopic Findings | Group A Number of Ears (40) & % | Group B Number of Ears (40) & % | X2 | P-value |
|---|---|---|---|---|
| Retracted tympanic membrane | 32 (80%) | 34 (85%) | 1.28 | >0.05 |
| Opaque tympanic membrane | 30 (75%) | 33 (82.5%) | ||
| Air fluid level | 3 (7.5%) | 2 (5%) | ||
| Air bubbles | 2 (5%) | 2 (5%) |
Table 5: Otoscopic examination at the end of 2nd post-treatment week.
| Tympanogram Type | Group A Number of Ears (40) & % | Group B Number of Ears (40) & % | X2 | P-value |
|---|---|---|---|---|
| A | 6 | 4 | 2,352 | >0.05 |
| C | 8 | 4 | ||
| B | 26 | 32 |
Table 6: Tympanometric results at the end of 2nd post-treatment week.
| Pure tone hearing threshold means ± SD | Independent samples t-test | P-value | |
|---|---|---|---|
| Group A | Group B | ||
| 23.1 ± 1.41 | 23.85 ± 1.84 | 1.445 | 0.157 |
Table 7: Pure tone hearing threshold average within 0, 5, 1, 2, and 4 KHz means at the end of 2nd post-treatment week.
Discussion
OME is a very common disease in children, and it is the leading cause of acquired hearing impairment during childhood. OME can occur as a result of Eustachian tube dysfunction. Adenoid hypertrophy, upper respiratory tract infection, allergy, and anatomical anomalies involving the palate and skull base are considered as causes of OME. Till now, there is controversy about the effectiveness of medical treatment, e.g. steroids, antibiotics, antihistamines, etc. in OME. Furthermore, the use of drugs is not free of side effects and cost issues. Therefore, the present study has addressed the efficacy of topical nasal steroid (mometasone) in the treatment of OME.
The current study has shown that there was no significant advantage of using mometasone nasal spray for the treatment of children with OME in comparison to saline nasal spray (P value >0.05). Perhaps, this result can be explained by the basis that intranasal steroids would not be expected to reach the middle ear.
William, in his study, found that intranasal steroid is unlikely to be of benefit in the treatment of children with OME [11] Nunes-Batalla et al. concluded that Topical corticoids are not advised for the treatment of OME patients since they have not been shown to have a substantial impact on the outcomes of OME [12]. Williamson et al. assessed the clinical efficacy of intranasal steroids in treating children with OME in general practice and concluded that these drugs are not likely to be clinically helpful [13]. In Vanneste et al. study, they stated that while some drugs, such as intranasal steroids, can be used to treat OME, these drugs are rarely consistently successful and rarely offer long-term relief [14]. “However, the preponderance of data suggests that intranasal steroids do not improve the longterm clearance of isolated OME and are therefore not recommended” [15]. In a systematic review done by Butler and Van der Voort et al., the authors found; in short-term follow-up studies, that oral or intranasal steroids, alone or in combination with antibiotics, can hasten the resolution of OME, but there is no evidence of a long-term benefit. Therefore, these drugs are not recommended for the treatment of OME [16].
On the other hand, in a study conducted by Cengle and Akyol, they found that intranasal mometasone furoate monohydrate is a useful alternative to surgery, at least in the short-term, for the treatment of OME [17]. A similar result was obtained by Kadah et al., and El-Anwar et al., who mentioned that topical nasal steroids are effective in the treatment of OME in the short term [18, 19].
However, the international recommendation against using steroids to treat OME is because of the side effects, cost issues, and no convincing evidence of long-term effectiveness [20]. The Cochrane review update on oral and intranasal steroids for the treatment of OME found no significant benefit of intranasal steroids [21].
Conclusion
In conclusion, topical nasal steroid, in form of mometasone furoate, has no significant benefit in the treatment of children with OME in short-term follow-up. Further studies for longer duration are recommended to evaluate the usefulness of long-term intranasal steroids for the treatment of OME.
References
- Emmett SD, Kokesh J, Kaylie D. Chronic ear disease. Med Clin. 2018;102(6):1063-79.
- Zernotti ME, Pawankar R, Ansotegui I, Badellino H, Croce JS, Hossny E, et al. Otitis media with effusion and atopy: Is there a causal relationship?. World Allergy Organ J. 2017;10:1-9.
- Tomonaga K, Kurono Y, Chaen T, Mogi G. Adenoids and otitis media with effusion: nasopharyngeal flora. Am J Otolaryngol. 1989;10(3):204-7.
- Nemade SV, Shinde KJ, Rangankar VP, Bhole P. Evaluation and significance of Eustachian tube angles and pretympanic diameter in HRCT temporal bone of patients with chronic otitis media. World J Otorhinolaryngol Head Neck Surg. 2018;4(4):240-5.
- Ghadersohi S, Ida JB, Bhushan B, Billings KR. Outcomes of tympanoplasty in children with down syndrome. Int J Pediatr Otorhinolaryngol. 2017;103:36-40.
- Kucur C, Simsek E, Kuduban O, Özbay İ. Prevalence of and risk factors for otitis media with effusion in primary school children: Case control study in Erzurum, Turkey. Turk J Pediatr. 2015;57(3):230.
- Chonmaitree T, Revai K, Grady JJ, Clos A, Patel JA, Nair S, et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46(6):815-23.
- Kasbekar AV, Patel V, Rubasinghe M, Srinivasan V. The surgical management of tympanic membrane retraction pockets using cartilage tympanoplasty. Indian J Otolaryngol Head Neck Surg. 2014;66:449-54.
- Cai T, McPherson B, Li C, Yang F. Pure tone hearing profiles in children with otitis media with effusion. Disabil Rehabil. 2018;40(10):1166-75.
- Teschner M. Evidence and evidence gaps in the treatment of Eustachian tube dysfunction and otitis media. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2016;15.
- Williamson I. Otitis media with effusion in children. BMJ Clin Evidence. 2015.
- Núñez-Batalla F, Jáudenes-Casaubón C, Sequí-Canet JM, Vivanco-Allende A, Zubicaray-Ugarteche J. Diagnosis and treatment of otitis media with effusion: CODEPEH recommendations. Acta Otorrinolaringol Esp. 2019;70(1):36-46.
- Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al. Topical intranasal corticosteroids in 4-11 year old children with persistent bilateral otitis media with effusion in primary care: Double blind randomised placebo controlled trial. BMJ. 2009;339.
- Vanneste P, Page C. Otitis media with effusion in children: Pathophysiology, diagnosis, and treatment. A review. J Otol. 2019;14(2):33-9.
- Roditi RE, Caradonna DS, Shin JJ. The proposed usage of intranasal steroids and antihistamines for otitis media with effusion.
Curr Allergy Asthma Rep. 2019;19:1-0. - Butler CC, van der Voort JH. Steroids for otitis media with effusion: A systematic review. Arch Pediatr Adolesc Med. 2001;155(6):641-7.
- Segal N, Leibovitz E, Dagan R, Leiberman A. Acute otitis media-diagnosis and treatment in the era of antibiotic resistant organisms: Updated clinical practice guidelines. Int J Pediatr Otorhinolaryngol. 2005;69(10):1311-9.
- Kadah SM, Elkholy TA, Tammam HK. Intranasal versus systemic corticosteroids in treatment of otitis media with effusion in the presence or absence of adenoid hypertrophy in children. The Egy J Otolaryngol. 2019;35:288-99.
- El-Anwar MW, Nofal AA, Khazbak AO, Sayed AE, Hassan MR. The efficacy of nasal steroids in treatment of otitis media with effusion: A comparative study. Int Arch Otorhinolaryngol. 2015;19:298-301.
- Simon F, Haggard M, Rosenfeld RM, Jia H, Peer S, Calmels MN, et al. International consensus (ICON) on management of otitis media with effusion in children. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1):S33-9.
- Simpson SA, Lewis R, van der Voort J, Butler CC. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2011(5).
Department of Surgery-Otolaryngology, College of Medicine, University of Baghdad, Baghdad, Iraq
Send correspondence to:
Ahmed Muhei Rasheed
Department of Surgery-Otolaryngology, College of Medicine, University of Baghdad, Baghdad, Iraq, E-mail: ahmed.muhei@comed.uobaghdad.edu.iq
Tel: 7713293222
Paper submitted on April 03, 2023; and Accepted on May 09, 2023
Citation: Rasheed AM. Is Topical Nasal Steroid Useful for Treatment of Otitis Media with Effusion in Children?. Int Tinnitus J. 2023;27(1):62-67.