The International Tinnitus Journal
Official Journal of the Neurootological and Equilibriometric Society
Official Journal of the Brazil Federal District Otorhinolaryngologist Society
ISSN: 0946-5448

Google scholar citation report
Citations : 12717
The International Tinnitus Journal received 12717 citations as per google scholar report
The International Tinnitus Journal peer review process verified at publons
Indexed In
- Excerpta Medica
- Scimago
- SCOPUS
- Publons
- EMBASE
- Google Scholar
- Euro Pub
- CAS Source Index (CASSI)
- Index Medicus
- Medline
- PubMed
- UGC
- EBSCO
Volume 28, Issue 1 / June 2024
Research Article Pages:122-128
10.5935/0946-5448.20240019
Suppression of Severe Tinnitus Via Acute Electrical Stimulation of The Round Window Niche: A Randomized Controlled Trial
Authors: Mario E. Zernotti, Giacomo Mandruzzato, Anandhan Dhanasingh, Mar�a Fernanda Di Gregorio, M�ximo Zernotti
PDF
Abstract
Introduction: At present, no current pharmacological or surgical interventions have been approved for the treatment of debilitating tinnitus. Since the late 1970s, it has been known that electrical stimulation of the cochlea can achieve partial or complete suppression of tinnitus percepts. This effect is also widely observed in users of cochlear implants. For individuals without concomitant severe-to-profound deafness, cochlear implantation is not a realistic option. Here we evaluated a promontory round window electrical stimulation device (PromStim, MED-EL, Innsbruck, Austria) as a potential treatment for severe tinnitus. Methods: 30 participants with grade 5 tinnitus were randomized into either an intervention or control group (n=15 each). All participants underwent myringotomy or micro flap surgery under local anesthesia. A stimulating electrode was placed at the round window niche. In the intervention group, stimulation was performed with biphasic pulses delivered at a rate of 100 or 5000 Hz. Stimulation was initially performed at an amplitude of 100 μA and incrementally increased. The control group underwent sham stimulation. The Tinnitus Handicap Inventory (THI) and a visual analog scale (VAS) of tinnitus severity were administered to all participants before and after the procedure. Results: In the intervention group, 9/15 participants (60%) had total suppression, 4/15 (26.6%) had partial suppression, and 2/15 (13.3%) had no suppression. In the control group, 2/15 participants (13.3%) had partial suppression and 13/15 (86.6%) had no suppression. After treatment, tinnitus severity was reduced to grade 4 in 10/15 participants (66.6%) in the intervention group and in 1/15 participants (6.6%) in the control group. Mean VAS scores of tinnitus severity were also reduced in the intervention group after stimulation. The mean duration of residual inhibition was 24.9 hours. Conclusion: Electrical stimulation of the round window niche can induce rapid and sustained suppression of tinnitus percepts, accompanied by reductions in self-reported severity. This may be a useful method for tinnitus control when cochlear implantation is not an option.
Keywords: Tinnitus, Electrophysiology, Electrical stimulation, Surgery, Audiology
Introduction
Tinnitus is the perception of sound in the absence of an external source. In extreme cases, tinnitus can have a severe negative impact on quality of life [1,2]. The commonly-used Tinnitus Handicap Inventory (THI) categorizes tinnitus into five grades of increasing severity, ranging from grade 1 (slight; when it is only heard in quiet environments, is very easily masked, and causes no interference with sleep or daily activities) to grade 5 (catastrophic; when it is always heard, disturbs sleep patterns, and causes difficulty with any activity). Tinnitus is common, being experienced by 12–30% of the world's adult population [3], with the prevalence of problematic and severe tinnitus reported to be 5% in adults and 3% in children [4,5]. To date, there are no approved pharmacological or surgical therapies to reduce the perception of tinnitus, or to treat its underlying cause (which is still a matter of considerable debate and research). Current interventions focus on psychological management strategies to reduce the stress and anxiety associated with problematic tinnitus such as cognitive behavioral therapy, acceptance and commitment therapy advice, and relaxation techniques. Sound therapies such as acoustic maskers have also been tested and shown to have some degree of efficacy.
An alternative method to reduce the perception of tinnitus is through electrical stimulation of the cochlea. The tinnitus suppressing effects of electrical stimulation delivered to the round window (RWES) have been known since the 1970s [6,7]. Although the mechanism behind this effect is incompletely understood, it is thought that stimulation can inhibit chronically hyperactive hair cells or neurons which generate abnormal auditory activity [8]. RWES is not approved as a stand-alone therapy, however tinnitus suppression has been widely observed in cochlear implant users, and these benefits can be quite large in magnitude and sustained over long durations [9-11]. Cochlear implants are indicated for only the most severe forms of sensorineural deafness and thus are not a realistic option for individuals without severe degrees of hearing impairment. The development of an RWES approach that does not rely on chronically implanted electrodes, cochlear or otherwise, may therefore be of great benefit for individuals suffering from debilitating tinnitus but who do not have concomitant sensorineural deafness.
Here we performed a randomized controlled trial to evaluate the clinical efficacy of RWES for suppression of severe (grade 5) tinnitus. The main objective of this study was to determine if tinnitus can be suppressed using a promontory electrical stimulation device (PromStim System, MED-EL, Innsbruck, Austria). This device employs a golf club type stimulating electrode which is placed acutely in the niche of the round window via a transtympanic route. Stimulation is managed using control devices and clinical software which are used for cochlear implant fitting and are thus readily available in many clinical audiological settings. We sought to determine the minimum thresholds of electrical stimulation required to achieve partial or total suppression of tinnitus, as well as the most effective stimulation rate. The secondary objective was to determine the duration of tinnitus suppression after stimulation is ceased (the residual inhibition, RI).
Materials and Methods
Study design, ethics, and informed consent: This was a prospective randomized analytical observational and interventional study. Participants were randomized into either the intervention or control group. Both groups underwent surgery for transtympanic electrode placement, but only the intervention group underwent electrical stimulation. This study was approved by the Institutional Health Research Ethics Committee of Sanatorio Allende (approval number: CIEIS-RePIS n2107-22). All participants gave their expressed signed informed consent before the start of any study related procedures.
Inclusion and exclusion criteria: The inclusion criteria for this study were adults (≥18 years) with catastrophic tinnitus (grade 5 on the THI instrument) who had no hearing loss or mild hearing loss (pure tone average thresholds lower than 20 dB HL or 45 dB HL, respectively, measured from 500–4000 Hz) and with measurable distortion product otoacoustic emissions or transitory evoked otoacoustic emissions. The exclusion criteria were the presence of central or retrocochlear disturbances; alcohol consumption (more than a glass of wine during the prior 24 hours); the use of melatonin, alprazolam, clonazepam, or any substance used to induce sleep; cigarette smoking during the test; or listening to loud music prior to the study. A prior history of treatment for tinnitus was not an exclusion criterion, but a washout period of at least one month following any drug treatment was a pre-requisite.
Surgery, electrode placement, and stimulation: After local infiltrative anesthesia with 4% lidocaine, all participants underwent a myringotomy or a micro flap surgery in the skin of the external auditory canal to access and visualize the round window niche. Under microscopy or endoscopy, the PromStim stimulating electrode (MED-EL, Innsbruck, Austria) was placed at the niche of the round window. The ground and reference were disposable surface electrodes. One was placed in the preauricular region and the other was placed in the angle of the mandible. The electrodes were connected to a Stimulation Box which was then connected to a MAX programming interface, which was then connected to a PC with the clinical stimulation software MAESTRO (all MED-EL).
Participants in the control group underwent myringotomy or a micro flap surgery and electrode placement, but no stimulation was performed. For participants in the intervention group, stimulation was controlled using the EABR task of the MAESTRO clinical stimulation software. The stimuli consisted of alternating biphasic pulses with a phase duration of 40 µs which were presented in a semi-continuous stimulation pattern. Based on prior studies, two different stimulation rates were tested: a low rate of 100 Hz [12] and a high rate of 5000 Hz [13].
Stimulation was initially performed at a stimulus level of 100 µA. The level was then increased in increments of 100 µA until either tinnitus suppression or an auditory response was observed. This procedure was repeated for both stimulation rates. The order in which the two stimulation rates were tested was randomized. If a level was found at which tinnitus suppression occurred, stimulation was continued at this level for 10 minutes. After this, the stimulation was reset to 100 µA and the second stimulation frequency was tested, followed by a 10 minute stimulation period if an effective suppression level was found. The participant was asked to report verbally if, during the stimulation, their tinnitus had diminished or been completely suppressed. The duration of RI was measured starting from the end of this stimulation period.
If no tinnitus suppression or auditory response was observed at the maximum permissible stimulation level of 1000 µA, or if the participant expressed discomfort or painful sensations, stimulation was discontinued, and the participant was considered untreatable.
Audiometric and patient reported measures: Three timepoints were selected for analysis: prior to stimulation (T0), immediately after the stimulation (T1), and at one week post-stimulation (T2).
At T0, all participants underwent pure tone audiometry to measure the pure tone average thresholds from 500–4000 Hz and the measurement of the frequency (Hz) and intensity (dB) of their tinnitus percepts through pitch matching and intensity matching, respectively.
For the patient reported measures, the validated Spanish version of the Tinnitus Handicap Inventory (THI) and a Visual Analogue Scale (VAS) [14] of perceived tinnitus intensity were administered. Both were administered at T0, T1, and via a telephone interview at T2. During the telephone interview, the participant was also asked to report if the tinnitus had returned during or after stimulation, and if so, when it had returned. From this, the RI was calculated.
Statistical analysis: For descriptive statistics, group mean values, Standard Deviations (SD), and ranges are used to summarize group-level data. Absolute frequencies and percentages are also given, when appropriate. For inferential statistics, a p-value of 0.05 was set as the threshold for significance. Wilcoxon signed-rank tests were used to assess the statistical significance of differences in VAS scores between different timepoints. The Chi-squared (χ2) test was used to assess the statistical significance of differences in the degree of tinnitus suppression with different stimulation rates. The McNemar test with χ2 was used to assess whether there was a significant effect of stimulation rate presentation order on VAS scores or on the RI. A permutation test was used to compare the mean stimulation levels required to achieve suppression across different stimulation rates.
Results
In total, 30 patients (8 female) participated in this study. Participants were randomized into a control group (n=15, 5 female) and an intervention group (n=15, 8 female). The mean (SD) age was 51.6 (±15.9) years in the control group and 47.7 (±15.4) years in the intervention group. The mean pure tone averages were 37.8 dB (±10.8, range: 10–50 dB) for the control group and 25.7 dB HL (±11.7, range: 10–45 dB) for the intervention group.
The mean tinnitus frequency was 5.7 (±2.0, range: 4–8) KHz and the mean tinnitus intensity was 59.0 (±18.6, range: 25–95) dB for the control group. The mean tinnitus frequency was 5.5 (±1.9, range: 4–8) KHz and the mean tinnitus intensity was 66.7 (±14.0; range: 40–80) dB for the intervention group. Individual data for the intervention group are provided in (Table 1).
| ID | Sex | Age at testing (years) | Audiometric PTA (dB HL) | Tinnitus frequency (kHz) | Tinnitus magnitude (dB) |
|---|---|---|---|---|---|
| 1 | M | 47.4 | 10 | 8 | 45 |
| 2 | M | 66 | 40 | 4 | 75 |
| 3 | M | 28.8 | 40 | 8 | 70 |
| 4 | F | 55.8 | 30 | 4 | 70 |
| 5 | M | 47.7 | 15 | 4 | 50 |
| 6 | M | 29.9 | 15 | 8 | 50 |
| 7 | M | 46.8 | 37 | 4 | 60 |
| 8 | F | 60.4 | 37 | 6 | 75 |
| 9 | M | 60.5 | 20 | 4 | 80 |
| 10 | M | 57.3 | 17 | 4 | 75 |
| 11 | M | 30.5 | 15 | 4 | 80 |
| 12 | M | 42.2 | 45 | 8 | 80 |
| 13 | F | 52.2 | 20 | 8 | 40 |
| 14 | M | 72 | 30 | 4 | 70 |
| 15 | M | 17.8 | 15 | 4 | 80 |
Table 1: Individual demographic and clinical data for the intervention group. PTA: Pure Tone Average at 500–4000 Hz
Responses to testing were classified into 3 categories: total suppression, partial suppression, and no suppression. In the intervention group, 9/15 participants (60%) had total suppression of tinnitus during the test, 4/15 (26.6%) had partial suppression, and 2/15 (13.3%) had no suppression. In the control group, 2/15 participants (13.3%) had partial suppression and 13/15 (86.6%) had no suppression.
Prior to testing, all participants had grade 5 tinnitus according to the THI. In the intervention group, tinnitus was reduced to grade 4 in 10/15 participants (66.6%) after stimulation. The other five participants (33.3%) remained at grade 5. In the control group, tinnitus was reduced to grade 4 in 1/15 participants (6.6%) after sham stimulation and remained at grade 5 in the other 14 participants (93.3%).
Stimulation or sham stimulation was suspended in two participants, one from each group, due to reported intense pain.
In the intervention group, mean (±SD) VAS scores were 9.1 (±0.6) points at T0, 2.0 (±2.7) points at T1, and 6.7 (±1.6) points at T2 (Figure 1A). The mean reduction in VAS scores between T0 and T1 was 7.1 (±3.1) points. This reduction was statistically significant (Wilcoxon signed-rank, z = 3.40, p = 0.0006). The mean reduction in VAS scores between T0 and T2 was 2.4 (±1.8) points. This reduction was statistically significant (Wilcoxon signed-rank, z=2.93, p = 0.0034). Seven participants in the intervention group achieved VAS of 0 points at the T1 timepoint.
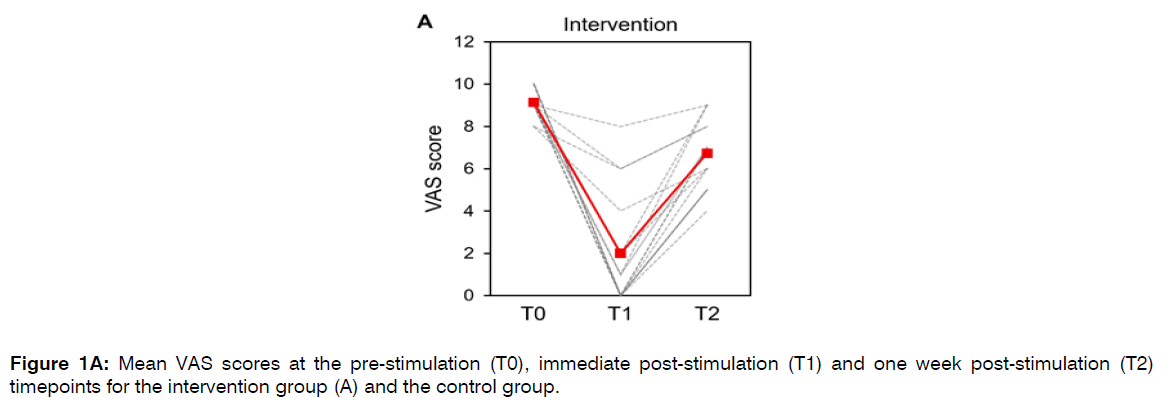
Figure 1A: Mean VAS scores at the pre-stimulation (T0), immediate post-stimulation (T1) and one week post-stimulation (T2) timepoints for the intervention group (A) and the control group.
In the control group, mean (±SD) VAS scores were 9.3 (±0.8) points at T0, 9.1 (±1.2) points at T1, and 9.0 (±1.1) points at T2 (Figure 1B). The mean reduction in VAS scores between T0 and T1 was 0.2 (±0.6) points. The mean reduction in VAS scores between T0 and T2 was 0.3 (0.6) points. Due to the large number of ties between the T0 and T1 timepoints and between the T0 and T2 timepoints (all participants had VAS scores of 7 or greater at all timepoints), the statistical significance of these comparisons could not be reliably calculated. Six participants in the control group had VAS scores of 10 points at all three timepoints.
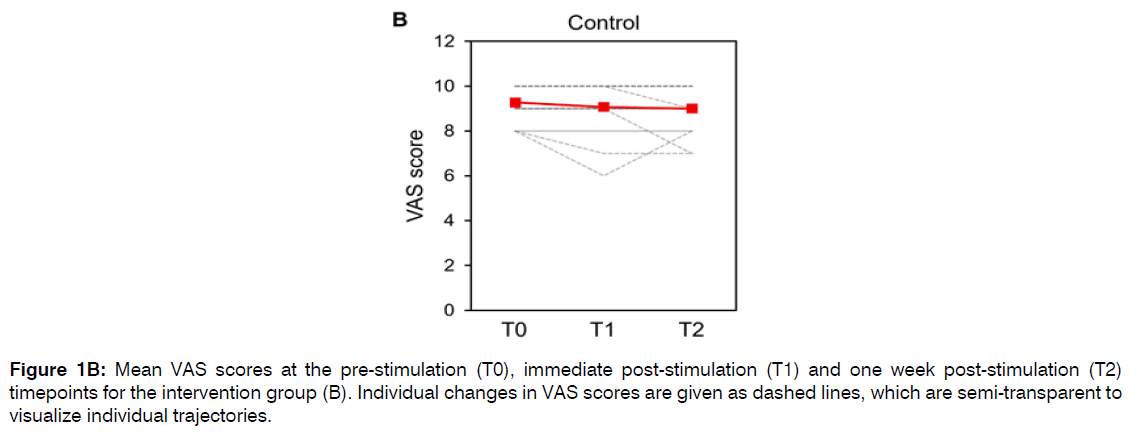
Figure 1B: Mean VAS scores at the pre-stimulation (T0), immediate post-stimulation (T1) and one week post-stimulation (T2) timepoints for the intervention group (B). Individual changes in VAS scores are given as dashed lines, which are semi-transparent to visualize individual trajectories.
The mean RI in the intervention group was 22.1 (±17.5, range: 0–48) hours. Neither of the two participants in the control group reported experiencing RI.
Comparing the 100 Hz vs 5000 Hz stimulation rates, there was not a significant difference in the degree of tinnitus suppression at the first stimulation (χ2, p > 0.05) nor was there an effect of presentation order of the stimulation type on VAS scores or on the RI (McNemar test with χ2, p = 0.683).
Across both stimulation sessions, the mean (±SD) stimulation level required to achieve any degree of suppression with the 100 Hz stimulation rate was 500 (±302.3, range: 200–1000) µA (Figure 2). The mean (±SD) stimulation level required to achieve any degree of suppression with the 5000 Hz stimulation rate was 654.2 (±295.0, range: 200–1000) µA. This difference was not statistically significant (permutation test p-value = 0.304).
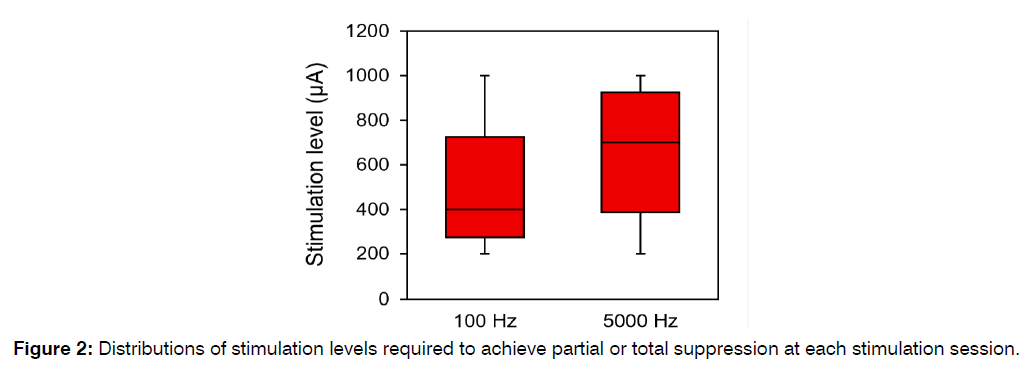
Figure 2: Distributions of stimulation levels required to achieve partial or total suppression at each stimulation session.
Discussion
Here we reported a randomized controlled trial of RWES for the suppression of grade 5 tinnitus. We observed that electrical stimulation at the round window niche under local anesthesia was able to suppress tinnitus in the majority of participants. In the intervention group, 9/15 participants (60%) experienced total suppression and 4/15 (27%) experienced partial suppression. Two participants (13%) experienced no suppression. Due to the subjective nature of tinnitus, it is possible that placebo effects may play a role in any observed suppression. For this reason, we also used a control group in which surgery and electrode placement were performed, but who underwent no RWES. In the control group, 2/15 participants experienced suppression, in both cases it was partial. These data would seem to provide evidence for a genuine effect of electrical stimulation.
Our findings that electrical stimulation of the round window niche can yield benefits for individuals suffering from tinnitus are largely in accord with prior literature. A recent systematic review by Assouly et al. examined 25 studies involving 1109 participants who underwent extracochlear electrical stimulation for tinnitus [14]. All studies found subjective improvement in tinnitus perception during or after electrical stimulation, and a wide range of different stimulation parameters were reported. The weight of evidence thus suggests that this is a viable and effective therapeutic option for this condition.
The tinnitus suppressing effects of cochlear electrical stimulation were discovered in the 1970s by researchers who were attempting to induce hearing percepts in patients with severe or profound deafness [15,16]. Portmann et al. published a case series of 15 participants where they applied RWES. It was found that the application of negative-polarity pulse trains induced auditory percepts, while positive-polarity pulse trains induced tinnitus suppression. They observed a rather large spread in the threshold stimulation levels required to achieve suppression in different participants (25–500 µA), as well as in the most effect stimulation rates (50–1600 Hz). It was noted that in all cases there was no RI, i.e., the suppression effect ceased as soon as electrical stimulation was stopped. This stands in contrast to the findings of the present study, which found that suppression typically endured for many hours after stimulation was ended (our mean RI was 22.1 hours, and suppression for 48 hours was achieved in one case). The cause of this difference in outcomes is unclear. The main methodological difference between our study and Portmann et al. is that we employed alternating biphasic pulses rather than positive polarity pulses. Charge balanced biphasic pulses are the standard type of stimulation used in cochlear implants today, as this type of stimulation prevents charge buildup within the cochlea.
Regarding stimulation rate, we did not observe a noteworthy difference between the low (100 Hz) and high (5000 Hz) stimulation rates in the incidence of suppression. This suggest that, at least on a group level, stimulation rate is not a dominant factor affecting the success of the procedure. Different stimulation rates have been reported across in prior studies. In a single-case study of a cochlear implant user, Zeng et al. [17] used low-rate stimulation (<100 Hz) with extracochlear stimulation of the round window niche, similar to the present study. It was found that stimulation at a high rate of 5000 Hz (on the same electrode) was ineffective at suppression. This suggest that, on the individual level, some stimulation rates may be more effective than others. Rubinstein et al. studied RWES in a group of eleven individuals with bothersome tinnitus and high-frequency hearing loss. It was found that high-rate stimulation at 4800 Hz was sufficient to induce partial or complete tinnitus suppression in 5/11 participants (45%) [15]. Other stimulation rates were not tested in that study, so the comparative efficacy of different stimulation rates could not be compared. In the present study, stimulation rates of 100 Hz and 5000 Hz were tested and no statistically significant difference in efficacy was found between the two stimulation rates.
Regarding the stimulus level, individuals in our study required a mean stimulus level of 500 µA at 100 Hz and 654.2 µA at 5000 Hz to achieve partial or total suppression. There was a large spread in this required level (200–1000 µA for both stimulation rates). This suggests a great deal of inter-individual variation, suggesting that stimulation levels may have to be determined empirically on an individual basis. Rubinstein et al. [11] achieved partial or complete tinnitus suppression in 5/11 participants who underwent RWES via myringotomy. The stimulus levels required to achieve suppression ranged approximately from 750 to 1500 µA in three of these participants. One participant who achieved tinnitus masking (i.e., an evoked auditory percept that masked the tinnitus percept) achieved this with a stimulation level of approximately 1200 µA. Portmann et al. [15] achieved suppression with a stimulation level as low as 25 µA in one participant, although this study used monophasic shaped pulses, making it less comparable with the present work.
The extent and degree of any RI is an important characteristic of any tinnitus suppression therapy. This determines the nature of any medical application derived from this research (i.e., how frequently RWES must be applied). The mean RI in our study was 24.9 hours (range: 0–48 hours). Rubinstein et al. [11] showed a RI ranging from 45 minutes to 72 hours in four of their five participants who achieved suppression. Based on these two studies, we can tentatively conclude that biphasic RWES can achieve post-stimulation RI of tinnitus which lasts from minutes to days.
The exact mechanism of tinnitus suppression with electrical stimulation is currently not well understood. One model suggests that neural degeneration within the cochlea leads to a compensatory hyperactivity in central auditory pathways, leading to the generation of tinnitus percepts. This model is supported by a recent study which found that in individuals with tinnitus and audiometrically normal hearing, cochlear neural degeneration could be observed in the form of reduced amplitudes of click-evoked action potentials measured via electrocochleography [12]. This was accompanied by increased amplitudes of post-Wave I components of the auditory brainstem response, consistent with hyperactive generators in the central auditory pathways. Within this framework, it is possible that electrical stimulation of the cochlea may act to impede this abnormal hyperactivity.
One limitation of the present study is that we did not investigate the site of origination of tinnitus (peripheral versus central). Aran and Cazals pointed out that electrical stimulation is only beneficial if the tinnitus is of peripheral origin [14]. If correct, this could be seen as a limitation of the RWES approach. Alternative methodologies may be required for the treatment of tinnitus of central origin.
Conclusion
Electrical stimulation of the round window niche can induce rapid and sustained suppression of tinnitus. However, we observed a relatively high degree of inter-individual variation in the magnitude of tinnitus suppression and in the duration of residual inhibition. More detailed studies will be performed to elucidate the roles of different stimulation parameters and determine which clinical and demographic variables influence these outcomes.
Conflicts of Interest statement
Mario Zernotti, Maria Fernanda Di Gregorio and Maximo Zernotti declare no conflicts of interest.
Anandhan Dhanasingh and Giacomo Mandruzzato work at MED-EL Medical Electronics GmbH, Innsbruck, Austria
Financial Support & Sponsorship
The PromStim System sets were provided by MED-EL Medical Electronics GmbH, Innsbruck, Austria
Acknowledgements
The authors would like to express their gratitude to the patients who agreed to participate in this research. The authors would also like to thank P. Connolly (MED-EL) for English language assistance with this manuscript.
References
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600-7.
- Baguley DM, Caimino C, Gilles A, Jacquemin L. The International Vocabulary of Tinnitus. Front Neurol. 2022;16:887592.
- Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959-65.
- Davis A, El Rafaie A. Epidemiology of tinnitus. Tinnitus Handbook Singular Pub. 2000: 1-25.
- Rosing SN, Schmidt JH, Wedderkopp N, Baguley DM. Prevalence of tinnitus and hyperacusis in children and adolescents: a systematic review. BMJ Open. 2016;6(6):e010596.
- Tyler RS, Rubinstein J, Pan T, Chang SA, Gogel SA, Gehringer A, et al. Electrical stimulation of the cochlea to reduce tinnitus. Semin Hear. 2008;29(04):326-32.
- Buechner A, Brendel M, Lesinski-Schiedat A, Wenzel G, Frohne-Buechner C, Jaeger B, et al. Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol Neurotol. 2010;31(9):1381-5.
- Ramos Á, Polo R, Masgoret E, Artiles O, Lisner I, Zaballos ML, et al. Cochlear implant in patients with sudden unilateral sensorineural hearing loss and associated tinnitus. Acta Otorrinolaringol. 2012;63(1):15-20.
- Portmann M, Cazals Y, Negrevergne M, Aran JM. Temporary tinnitus suppression in man through electrical stimulation of the cochlea. Acta Otorrinolaringol. 1979;87(3-6):294-9.
- Zeng FG, Tang Q, Dimitrijevic A, Starr A, Larky J, Blevins NH. Tinnitus suppression by low-rate electric stimulation and its electrophysiological mechanisms. Hear Res. 2011;277(1-2):61-6.
- Rubinstein JT, Tyler RS, Johnson A, Brown CJ. Electrical suppression of tinnitus with high-rate pulse trains. Otol Neurotol. 2003;24(3):478-85.
- Vasilkov V, Caswell-Midwinter B, Zhao Y, de Gruttola V, Jung DH, Liberman MC, et al. Evidence of cochlear neural degeneration in normal-hearing subjects with tinnitus. Sci Rep. 2023;13(1):19870.
- Assouly KK, Dullaart MJ, Stokroos RJ, van Dijk B, Stegeman I, Smit AL. Systematic review on intra-and extracochlear electrical stimulation for tinnitus. Brain sciences. 2021;11(11):1394.
- Aran JM, Cazals Y. Electrical suppression of tinnitus. Tinnitus. 1981:217-31.
- Cazals Y, Negrevergne M, Aran JM. Electrical stimulation of the cochlea in man: hearing induction and tinnitus suppression. Ear Hear. 1978;3(5):209-13.
- Peter N, Liyanage N, Pfiffner F, Huber A, Kleinjung T. The influence of cochlear implantation on tinnitus in patients with single-sided deafness: a systematic review. World J Otorhinolaryngol Head Neck Surg. 2019;161(4):576-88.
- Sirh SJ, Sirh SW, Mun HY, Sirh HM. Integrative treatment for tinnitus combining repeated facial and auriculotemporal nerve blocks with stimulation of auditory and non-auditory nerves. Front Neurol. 2022;16:758575.
1Department of Otorhinolaryngology, Sanatorio Allende de Córdoba, Córdoba, Argentina
2MED-EL Medical Electronics, Innsbruck, Austria
Send correspondence to:
Mario E. Zernotti
Department of Otorhinolaryngology, Sanatorio Allende de Córdoba, Córdoba, Argentina, E-mail: mario.zernotti@gmail.com
Paper submitted on April 19, 2024; and Accepted on June 03, 2024
Citation: Mario E. Zernotti. Suppression of Severe Tinnitus via Acute Electrical Stimulation of the Round Window Niche: A Randomized Controlled Trial. Int Tinnitus J. 2024;28(1):122-128.


